Deborah A. Brown, Ph.D.

Professor
Department of Biochemistry and Cell Biology
Life Sciences Building
Stony Brook University
Stony Brook, NY 11794-5215
Phone: 631-632-8563
E-mail: deborah.brown@stonybrook.edu
- Research Description
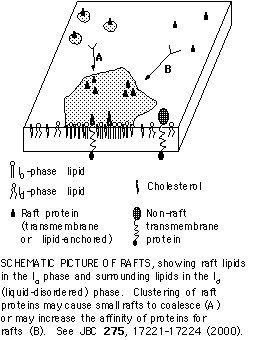 Recent work has given rise to a fundamentally new way of thinking about biological
membranes. Contrary to earlier views, we now know that lipids do not always mix homogeneously
in membranes, but can be organized into domains in the bilayer. The best-characterized
membrane domains, called rafts, are rich in cholesterol and sphingolipids. The ability
of certain proteins to associate with these domains has profound effects on their
function. This new realization has implications in fields as diverse as signal transduction,
protein and lipid sorting, and cell adhesion and motility. For instance, following
antigen stimulation of T lymphocytes, the T cell receptor and its signaling partners
must cluster together in rafts to initiate the signal transduction cascade.
Recent work has given rise to a fundamentally new way of thinking about biological
membranes. Contrary to earlier views, we now know that lipids do not always mix homogeneously
in membranes, but can be organized into domains in the bilayer. The best-characterized
membrane domains, called rafts, are rich in cholesterol and sphingolipids. The ability
of certain proteins to associate with these domains has profound effects on their
function. This new realization has implications in fields as diverse as signal transduction,
protein and lipid sorting, and cell adhesion and motility. For instance, following
antigen stimulation of T lymphocytes, the T cell receptor and its signaling partners
must cluster together in rafts to initiate the signal transduction cascade.Our lab has developed a model for raft structure, and for how proteins and lipids associate with rafts, that is now generally accepted in the field. This model provides a conceptual foundation for further exploration of raft structure and function. We use a combination of cell biological, biochemical, and biophysical methods to study rafts in cells and model membranes.
Raft Structure
The acyl chains of raft lipids are highly extended and tightly packed. This arrangement gives raft lipids a high degree of order. This contrasts with the disordered state of most lipids in biological membranes. In fact, rafts probably exist in a separate phase from the rest of the membrane, that has properties similar to those of the liquid-ordered (lo) phase described in model membranes. Lipids such as sphingolipids, which have long, saturated acyl chains, partition preferentially into these ordered domains and are enriched in rafts.
A number of proteins that are modified with saturated acyl chains (including glycosyl phosphatidylinositol (GPI)-anchored proteins, myristoylated, and palmitoylated proteins) are enriched in rafts. These include important signaling proteins, such as Src-family kinases and heterotrimeric G protein alpha subunits. Association of these proteins with rafts is likely to be important in function, as has already been shown for the Src-family kinase Lck in T cells.
Current projects
Questions of raft structure are a major focus of our lab. How big
 are rafts? How are they distributed in membranes? How does clustering of raft proteins
(as can occur during signaling through cell-surface receptors) affect raft structure
and function? How are transmembrane proteins, which might not be expected to fit well
into an ordered lipid environment, targeted to rafts in the absence of acylation?
A close collaboration with the lab of Dr. Erwin London, a membrane biophysical chemist,
allows us to combine complementary cell biological, biochemical, and biophysical methods.
This powerful approach gives us a unique advantage in studying rafts in both cells
and model membranes.
are rafts? How are they distributed in membranes? How does clustering of raft proteins
(as can occur during signaling through cell-surface receptors) affect raft structure
and function? How are transmembrane proteins, which might not be expected to fit well
into an ordered lipid environment, targeted to rafts in the absence of acylation?
A close collaboration with the lab of Dr. Erwin London, a membrane biophysical chemist,
allows us to combine complementary cell biological, biochemical, and biophysical methods.
This powerful approach gives us a unique advantage in studying rafts in both cells
and model membranes.Caveolae
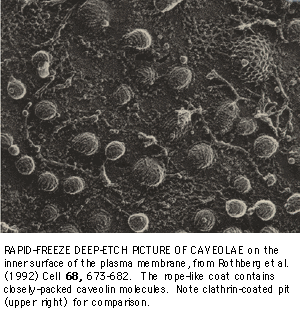
Caveolae are 50-100 nm pits in the plasma membrane of many mammalian cells, that contain the 22 kDa protein caveolin as a major structural component. Though caveolae were first described more than 30 years ago, their function(s) in endocytosis, cholesterol trafficking, and/or as signal transduction centers are just now being defined. Rafts have an affinity for caveolae, although the precise relationship between the two remains an intriguing mystery.
Caveolin is an unusual protein. Cytoplasmic N- and C-terminal domains flank a central 33 amino acid hydrophobic domain, which is presumed to form a tight alpha-helical hairpin that anchors the protein in the membrane. The protein forms large homo-oligomers, that can be up to 600 kDa in size. Caveolin may bind directly to a number of signaling proteins, modulating their function. It also binds tightly to cholesterol, and has been reported to cycle between the plasma membrane and intracellular compartments in an unusual manner. Caveolin is also the only protein known to associate directly with rafts without lipid modification. These and other unusual properties focus attention on this protein in linking the functions of rafts and caveolae.
Current projects
We have purified 100-microgram amounts of caveolin from recombinant-baculovirus infected insect cells, and reconstituted it into liposomes for structural studies. We are also examining the behavior of over-expressed caveolin and of a number of caveolin mutant proteins in cells. Together, these experiments will shed new light on this intriguing protein, and how it may function to organize rafts in cells.
- Selected Publications
- Schroeder, R., London, E., and Brown, D. A. (1994) Interactions between saturated acyl chains confer detergent resistance on lipids and GPI-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc. Natl. Acad. Sci. USA 91: 12130-12134
- Melkonian, K. A., Chu, T., Tortorella, L. B., and Brown, D. A. (1995) Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry 34: 16161-16170
- Ahmed, S. N., Brown, D. A., and London, E. (1997) On the origin of sphingolipid-cholesterol rich detergent-insoluble domains in cell membranes: Physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 36: 10944-10953
- Schroeder, R. A., Ahmed, S. N., Zhu, Y., London, E., and Brown, D. A. (1998) Cholesterol and sphingolipid enhance the Triton X-100-insolubility of GPI-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J. Biol. Chem. 273: 1150-1157
- Arni, S., Keilbaugh, S. A., Ostermeyer, A. G., and Brown, D. A. (1998) Association of the neuronal protein GAP-43 with detergent-resistant membranes requires two palmitoylated cysteine residues. J. Biol. Chem. 273: 28478-28485
- Melkonian, K. A., Ostermeyer, A. G., Chen, J. Z., Roth, M. G., and Brown, D. A. (1999) Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts: Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274: 3910-3917
- Ostermeyer, A. G., Beckrich, B. T., Ivarson, K. A., Grove, K. E., and Brown, D. A. (1999) Glycosphingolipids are not essential for formation of detergent-resistant membrane rafts in melanoma cells METHYL-beta-CYCLODEXTRIN DOES NOT AFFECT CELL-SURFACE TRANSPORT OF A GPI-ANCHORED PROTEIN. J. Biol. Chem. 274: 34459-34466
- Moffett, S., Brown, D. A., and Linder, M. E. (2000) Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275: 2191-2198
Reviews
- Brown, D. A. and London, E. (1997) Structure of detergent-resistant membrane domains: Does phase separation occur in biological membranes? Biochem. Biophys. Res. Commun. 240: 1-7
- Brown, D. A., and London, E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Devel. Biol. 14: 111-136
- Brown, D. A., and London, E. (1998) Structure and origin of ordered lipid domains in biological membranes. J. Membr. Biol. 164: 103-114
- Brown, D. A., and London, E. (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275: 17221-17224