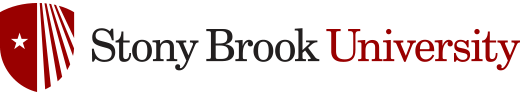Professor Reeder's research encompasses geochemistry and mineralogy, with focuses on environmental contamination, materials properties, and links to environmental health. Major goals of this work are to understand geochemically and environmentally important reaction mechanisms, especially those involving metal species, interactions at mineral surfaces, and bioavailability. Reeder's group makes extensive use of synchrotron X-ray facilities at nearby Brookhaven National Laboratory and at Argonne National Laboratory for studying metal speciation in sediments, soils, and aquatic systems, as well as structural properties of biomaterials and transformations in minerals.
Mineral-Water Interactions and Environmental Geochemistry
Structural and chemical properties of mineral surfaces provide some of the most fundamental constraints on reactions such as sorption, coprecipitation, crystal growth, and dissolution. Reeder's research program examines the structure and behavior of mineral surfaces in order to understand the mechanisms that control the fate of metals, including toxic species and radionuclides. A particular aspect of this work concerns the relationships among bulk crystal structure, surface structure, and metal uptake mechanisms. Various experimental techniques are used for correlating element partitioning with surface structure and growth mechanisms.
Trace elements that coprecipitate with a mineral also act as sensitive "probes" of surface sites. Research by Reeder's group on the mechanisms of trace element incorporation in carbonates, phosphates, and silicates has revealed the manner in which differences in surface structure affect trace element uptake and incorporation. Experiments involve both synthetic and natural samples, and address both the partitioning behavior and incorporation mechanisms of geochemically and environmentally important trace metals among structurally distinct surface sites, as well as the local coordination of metals on mineral surfaces and in bulk minerals. Synchrotron-based X-ray absorption spectroscopy allows direct characterization of the local environment of metal species, and synchrotron X-ray fluorescence microanalysis provides spatial distribution patterns for surface-controlled incorporation. This work has shown that the size and coordination geometry of sites as well as adsorption mechanisms are fundamental factors controlling element incorporation. The results of this work have been applied to trace element diagenetic studies of carbonate rocks, to trace element records of climate change, and to the uptake and mobility of toxic metals and radionuclides in environmentally sensitive areas.
Chemical Speciation of Heavy Metals and Bioavailability
 Some of the most serious contaminants in the environment are toxic metals and metalloids.
Common examples include lead, arsenic, chromium, mercury, and uranium. Their partitioning
between solid phases such as minerals and the aqueous phase is one of the dominant
factors controlling metal mobility and bioavailability in the environment. Prof.
Reeder’s group examines these processes from a molecular perspective, with a view
toward understanding chemical speciation of metals and the associated reaction mechanisms
in natural and contaminated systems. This work makes use of both lab and synchrotron-based
spectroscopic and scattering techniques, including XANES and EXAFS, which offer molecular-scale
information. Information gained from this research provides the fundamental basis
for remediation strategies for contaminated sites, as well as a direct link to the
fields of Medical Geology and human health.
Some of the most serious contaminants in the environment are toxic metals and metalloids.
Common examples include lead, arsenic, chromium, mercury, and uranium. Their partitioning
between solid phases such as minerals and the aqueous phase is one of the dominant
factors controlling metal mobility and bioavailability in the environment. Prof.
Reeder’s group examines these processes from a molecular perspective, with a view
toward understanding chemical speciation of metals and the associated reaction mechanisms
in natural and contaminated systems. This work makes use of both lab and synchrotron-based
spectroscopic and scattering techniques, including XANES and EXAFS, which offer molecular-scale
information. Information gained from this research provides the fundamental basis
for remediation strategies for contaminated sites, as well as a direct link to the
fields of Medical Geology and human health.
Biomineralization and Bio-inspired Synthesis
Geochemists often take cues from biology. One important example is biomineralization,
where various organisms form mineralized skeletal components having specific form
and function. Calcium carbonates and phosphates are common examples and occur in
a variety of vertebrate and invertebrate organisms. In the case of biogenic calcium carbonate, it is now widely understood that a crystalline form, such as calcite or
aragonite, is not usually the first solid that forms. The more common biomineralization
pathway is via formation of an amorphous calcium carbonate (ACC) precursor that subsequently
transforms to a crystalline phase. An advantage of this approach is the ability to
create a structural component, for example in a shell, having a particular shape intended
for a specific function; this is made possible because ACC lacks long-range order,
whereas crystals exhibit the tendency to grow in certain directions and with a specific
shape. This amorphous precursor strategy has inspired chemists and materials scientists
in designing novel functional materials. Inorganic amorphous calcium carbonate also
occurs naturally, and may be important intermediate phases in the carbon cycle.
carbonate, it is now widely understood that a crystalline form, such as calcite or
aragonite, is not usually the first solid that forms. The more common biomineralization
pathway is via formation of an amorphous calcium carbonate (ACC) precursor that subsequently
transforms to a crystalline phase. An advantage of this approach is the ability to
create a structural component, for example in a shell, having a particular shape intended
for a specific function; this is made possible because ACC lacks long-range order,
whereas crystals exhibit the tendency to grow in certain directions and with a specific
shape. This amorphous precursor strategy has inspired chemists and materials scientists
in designing novel functional materials. Inorganic amorphous calcium carbonate also
occurs naturally, and may be important intermediate phases in the carbon cycle.
Reeder's group, in collaboration with Prof. Brian Phillips, is studying the short-
and medium-range structure in amorphous biominerals and synthetic analogs using a
combination of spectroscopic and scattering techniques, including pair distribution
function analysis and X-ray absorption spectroscopy. Students in Reeder’s group are
studying the effects of chemical additives on ACC structure and its stability and
transformation kinetics. These results translate to a better understanding of biomineralization
mechanisms and bio-inspired synthesis.
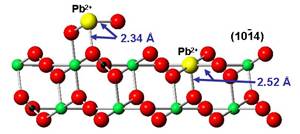
Particulate Matter and Lung Health
Inhalation of airborne particulate matter (PM) has long been recognized as contributing
to pulmonary impairment. In arid locations, mineral dust can be a significant component
of airborne PM. Reeder’s group has used synchrotron X-ray methods to characterize
the compositional and structural properties of 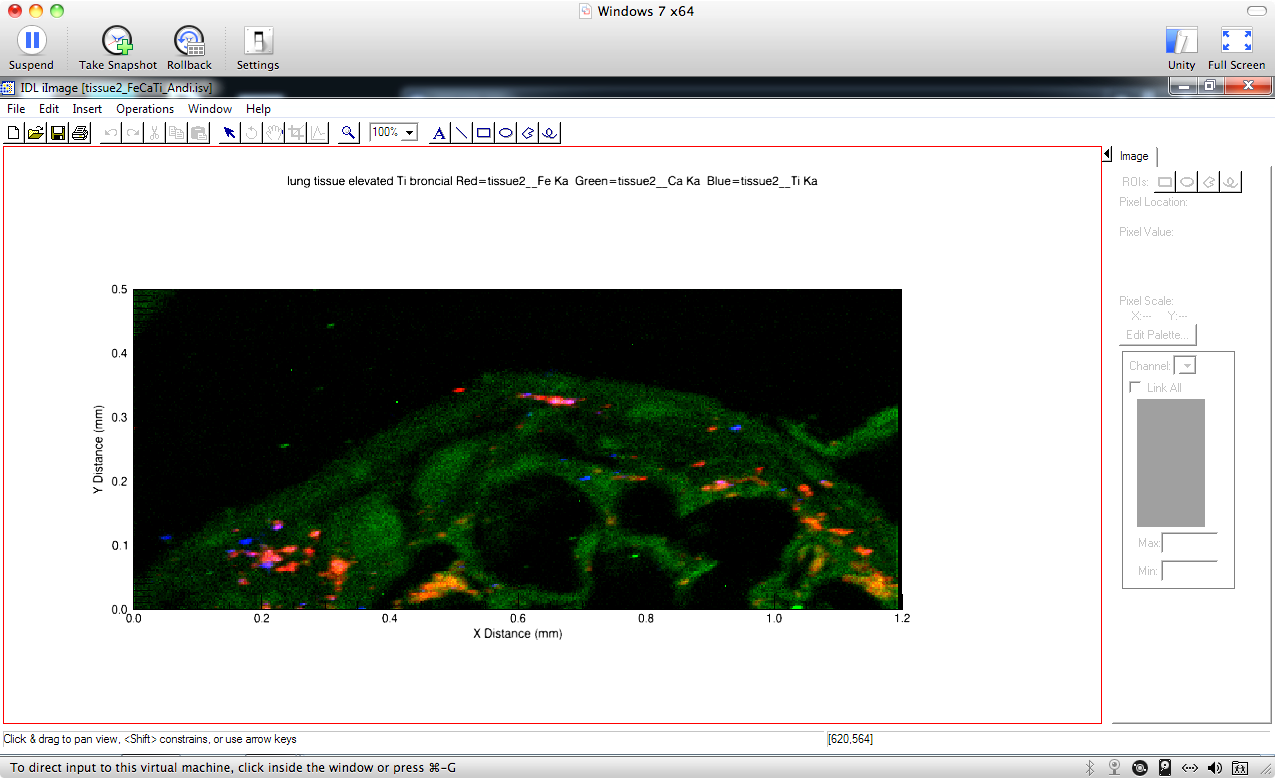 PM2.5 and PM10 from locations with known incidence of lung injury. The micro-focusing capability
at synchrotron beamlines allows chemical speciation of hazardous metals and metalloids
in small particles and correlation with bioavailability and lung injury. A particular
focus of Reeder’s group has been the extensive exposure of servicemen and women deployed
in the Iraq and Afghanistan conflicts to PM containing metals and other hazardous
substances. Using synchrotron micro-XRF/XAS, they have partnered with pulmonary researchers
to reveal that heavy metals and metalloids are present in particles in diseased lung
tissue (Figure), and these same metal species are found in airborne PM. Such studies
bring together geochemists and medical researchers to address emerging challenges
in environmental health.
PM2.5 and PM10 from locations with known incidence of lung injury. The micro-focusing capability
at synchrotron beamlines allows chemical speciation of hazardous metals and metalloids
in small particles and correlation with bioavailability and lung injury. A particular
focus of Reeder’s group has been the extensive exposure of servicemen and women deployed
in the Iraq and Afghanistan conflicts to PM containing metals and other hazardous
substances. Using synchrotron micro-XRF/XAS, they have partnered with pulmonary researchers
to reveal that heavy metals and metalloids are present in particles in diseased lung
tissue (Figure), and these same metal species are found in airborne PM. Such studies
bring together geochemists and medical researchers to address emerging challenges
in environmental health.
Crystal Chemistry of Carbonates and Phosphates
Related research pertains to structure and stability of minerals, particularly order-disorder and local structure in solid solutions in carbonates and phosphates. Many of the solids that form in surface environments are solid solutions or exhibit extensive substitution, which affects their stability and reactivity. This work combines X-ray scattering methods with EXAFS spectroscopy. Particular problems include substitution mechanisms and solid solution properties for structurally incompatible components. Other studies include studies of phase transitions in minerals and their influence on stability.
Selected Publications
Hur, H. and Reeder, R.J. (2016) Tungstate sorption mechanisms on boehmite: Systematic uptake studies and X-ray absorption spectroscopy analysis. J. Colloid. Interface Sci., 461, 249-260.
Sklute, E.C., Jensen, H.B., Rogers, A.D., Reeder, R.J. (2015) Morphological, structural, and spectral characteristics of amorphous iron sulfates, J. Geophys. Res. Planets, 120, 809-830.
Cerkez, E.B., Bhandari, N., Reeder, R.J., and Strongin, D.R. (2015) Coupled redox transformation of chromate and arsenite on ferrihydrite. Environ. Sci. Tech., 49, 2858-2866.
Szema, A.M., Reeder, R.J., Harrington, A.D., Schmidt, M., Liu, J., Golightly, M., Rueb, T., Hamidi, S.A., (2014) Iraq dust is respirable, sharp, and metal-laden and induces lung inflammation with fibrosis in mice via IL-2 upregulation and depletion of regulatory T cells. J. Occup. Environ. Med., 56, 243-251.
Schmidt, M.P., Ilott, A.J., Phillips, B.L., and Reeder, R.J. (2014) Structural changes upon dehydration of amorphous calcium carbonate. Cryst. Growth Design, 14, 938−951.
Reeder, R.J. and Michel, F.M. (2013) Application of total X-ray scattering methods and pair distribution function analysis for study of structure of biominerals. Methods in Enzymology, 532, 477-500.
Reeder, R.J., Tang, Y., Schmidt, M.P., Kubista, L.M., Cowan, D.F., Phillips, B.L. (2013) Characterization of structure in biogenic amorphous calcium carbonate: Pair distribution function and nuclear magnetic resonance studies of lobster gastrolith. Cryst. Growth Design, 13, 1905-1914.
Bhandari, N., Reeder, R.J., and Strongin, D.R. (2012) Photoinduced oxidation of arsenite to arsenate in the presence of goethite. Environ. Sci. Tech., 46, 8044-8051.
Szema, A.M, Schmidt, M.P., Lanzirotti, A., Harrington, A.D., Lyubsky, S., Reeder, R.J., Schoonen, M.A.A. (2012) Titanium and iron in lung of a soldier with nonspecific interstitial pneumonitis and bronchiolitis after returning from Iraq. J. Occup. Environ. Med., 54, 1-2.
Bhandari, N., Reeder, R.J., and Strongin, D.R. (2011) Photoinduced oxidation of arsenite to arsenate on ferrihydrite. Environ. Sci. Tech., 45, 2783-2789.
Tang, Y., Michel, F.M., Zhang, L., Harrington, R., Parise, J.B., and Reeder, R.J. (2010) Structural properties of the Cr(III)-Fe(III)-(oxy)hydroxide compositional series: Insights for nanomaterial “solid solution”. Chem. Materials, 22, 3589-3598.
Goodwin, A.L., Michel, F.M., Phillips, B.L., Keen, D.A., Dove, M.T., Reeder, R.J. (2010) Nanoporous structure and medium-range order in synthetic amorphous calcium carbonate. Chem. Materials, 22, 3197-3205.
Elzinga, E.J., Tang, Y., McDonald, J., DeSisto, S., and Reeder, R.J. (2009) Macroscopic and spectroscopic characterization of selenate, selenite, and chromate adsorption at the solid-water interface of γ-Al2O3. J. Colloid Interface Sci., 340, 153-159.
Lee, Y.J., Stephens, P.W., Tang, Y., Li, W., Phillips, B.L., Parise, J.B., and Reeder, R.J. (2009) Arsenate substitution in hydroxylapatite: Structural characterization of the Ca5(PxAs1-xO4)3OH solid solution. Am. Mineral., 94, 666-675.
Tang, Y., Chappell, H.F., Dove, M.T., Reeder, R.J., and Lee, Y.J. (2009) Zinc incorporation into hydroxylapatite. Biomaterials, 30, 2864-2872.
Tang, Y. and Reeder, R.J. (2009) Uranyl and arsenate cosorption on aluminum oxide surface. Geochim. Cosmochim. Acta, 73, 2727-2743.
Tang, Y. and Reeder, R.J. (2009) Enhanced uranium sorption on aluminum oxide pretreated with arsenate. Part I: Batch uptake behavior. Environ. Sci. Tech., 43, 4446-4451.
Tang, Y., McDonald, J., and Reeder, R.J. (2009) Enhanced uranium sorption on aluminum oxide pretreated with arsenate. Part II: Spectroscopic studies. Environ. Sci. Tech., 43, 4452-4458.
Michel, F.M., MacDonald, J. Feng, J., Phillips, B.L.,Ehm, L., Tarabrella, C., Parise,
J.B., and Reeder, R.J. (2008) Structural characteristics of synthetic amorphous calcium
carbonate. Chem. Materials, 20, 4720-4728.
Burns, F.J., Rossman, T., Vega, K., Uddin, A., Vogt, S., Lai, B., and Reeder, R.J.
(2008) Mechanism of selenium-induced inhibition of arsenic-enhanced UVR carcinogenesis
in mice. Environ. Health Perspectives, 116, 703-708.
Tang, Y., Elzinga, E.J, Lee, Y.J., and Reeder, R.J. (2007) Coprecipitation of chromate with calcite: Batch experiments and X-ray absorption spectroscopy. Geochim. Cosmochim. Acta, 71, 1480-1493.
Hausner, D.B., Reeder, R.J., and Strongin, D.R. (2007) Humidity-induced restructuring of the calcite surface and the effect of divalent heavy metals. J. Colloid Interface Sci., 305, 101-110.
Alexandratos, V.G., Elzinga, E.J., and Reeder, R.J. (2007) Arsenate uptake by calcite: Macroscopic and spectroscopic characterization of adsorption and incorporation mechanisms. Geochim. Cosmochim. Acta, 71, 4172-4187.
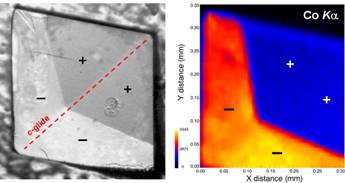
Rouff, A.A., Elzinga, E.J., Reeder, R.J., and Fisher, N.S. (2006) The effect of aging and pH on Pb(II) sorption processes at the calcite-water interface. Environ. Sci. Technol., 40, 1792-1798.
Lee, Y.J. and Reeder, R.J. (2006) The role of citrate and phthalate during Co(II) coprecipitation with calcite. Geochim. Cosmochim. Acta, 70, 2253-2263.
Elzinga, E.J., Rouff, A.A., and Reeder, R.J. (2006) The long-term fate of Cu2+, Zn2+, and Pb2+ adsorption complexes at the calcite surface: An X-ray absorption spectroscopy study. Geochim. Cosmochim. Acta, 70, 2715-2725.
Lee, Y.J., Elzinga, E.J, and Reeder, R.J. (2005) Cu(II) adsorption at the calcite-water interface in the presence of natural organic matter: kinetic studies and molecular-scale characterization. Geochim. Cosmochim. Acta 69, 49-61.
Lee, Y.J., Elzinga, E.J, and Reeder, R.J. (2005) Sorption mechanisms of zinc on hydroxyapatite: Systematic uptake studies and EXAFS spectroscopy analysis. Environ. Sci. Technol. 39, 4042-4048.
Phillips, B.L., Lee, Y.J., and Reeder, R.J. (2005) Organic coprecipitates with calcite: NMR spectroscopic evidence. Environ. Sci. Technol. 39, 4533-4539.
Rouff, A.A., Reeder, R.J., and Fisher, N.S. (2005) Electrolyte and pH effects on Pb(II)-calcite sorption processes: the role of the PbCO30(aq) complex. J. Colloid Interface Sci.,286, 61-67.
Chada, V.G.R., Hausner, D.B., Strongin, D.R., Rouff, A.A., and Reeder, R.J. (2005) Divalent Cd and Pb uptake on calcite {} cleavage faces: An XPS and AFM study. J. Colloid Interface Sci. 288, 35-360.
Rouff, A.A., Elzinga, E.J., Reeder, R.J., and Fisher, N.S. (2005) The Influence of pH on the kinetics, reversibility and mechanisms of Pb(II) sorption at the calcite-water interface. Geochim. Cosmochim. Acta 69, 5173-5186.