Enhanced Permeability and Retention (EPR) – based Tumor-Targeted Drug Delivery of Taxoids
The drug discovery program includes tumor-specific drug delivery systems, “guided molecular missiles” in the fight against cancer. Despite the significant progress in the development of cancer detection, prevention, surgery and therapy, there is still no common cure for this disease. In addition, the long-standing problem of chemotherapy is the lack of tumor-specific treatments. Traditional chemotherapy relies on the premise that rapidly proliferating cancer cells are more likely to be killed by a cytotoxic agent. In reality, however, cytotoxic agents have very little or no specificity, which leads to systemic toxicity, causing undesirable severe side effects. Therefore, various “molecularly targeted cancer therapies” have been developed for use in specific cancers, including tumor-targeting drug delivery (TTDD). In general, a TTDD system consists of a tumor recognition moiety and a cytotoxic warhead connected through a “smart” linker to form a conjugate. When a multi-functionalized nanomaterial is used as the vehicle, a “Trojan Horse” approach becomes possible for mass delivery of cytotoxic warheads to maximize the efficacy. The Ojima Laboratory has been making an excellent progress in the novel molecular approaches to the design and discovery of “guided molecular missiles” for tumor-targeting chemotherapy.
Taxoids as Potent Cytotoxic Agents for Tumor-Targeting Drug Delivery
One of the key problems with conventional chemotherapy is its toxicity: In addition to its beneficial characteristics of killing cancer cells, anticancer drugs also destroy healthy tissue resulting in systemic toxicity. Improvement in the potency and anti-MDR activities of anticancer drugs has provided efficient in treatment of aggressive and resistant tumors, although systemic toxicity persists and plays an important role in overall efficacy.
A Possible solution is Tumor-Targeting Drug Delivery which combines powerful anti-cancer compounds with tumor-targeting molecules. Paul Ehrlich (14 March 1854 – 20 August 1915) was a German scientist who won the 1908 Nobel Prize in Physiology or Medicine. He is noted for his work in hematology, immunology, and chemotherapy. Ehrlich predicted autoimmunity calling it "horror autotoxicus". He coined the term “chemotherapy" and popularized the concept of a “magic bullet". He is credited with the first empirical observation of the blood-brain barrier and the development of the first antibacterial drug in modern medicine.
ImmunoGen has developed technology known as “tumor-activiated prodrugs,” (TAPs), in which the anticancer agents are linked chemically to certain types of monoclonal antibodies. Those antibodies specifically bind to antigens that are known to be expressed predominantly on the cancer cell surface. The anit-cancer drug is inactive until it enters the tumor cell to which it is targeted to by the monoclonal antibody. In this way, the anticancer drugs are exclusively delivered to the cancer cells, leaving the normal cells intact. We have designed and synthesized novel taxanes used as cytotoxic agents in TAPs. Biological assays have since shown very impressive results.
S. Jaracz, J. Chen, L. V. Kuznetsova, and I. Ojima, “Recent Advances in Tumor-targeting Anticancer Drug Conjugates”, Bioorg. Med. Chem., 13, 5043-5054 (2005)
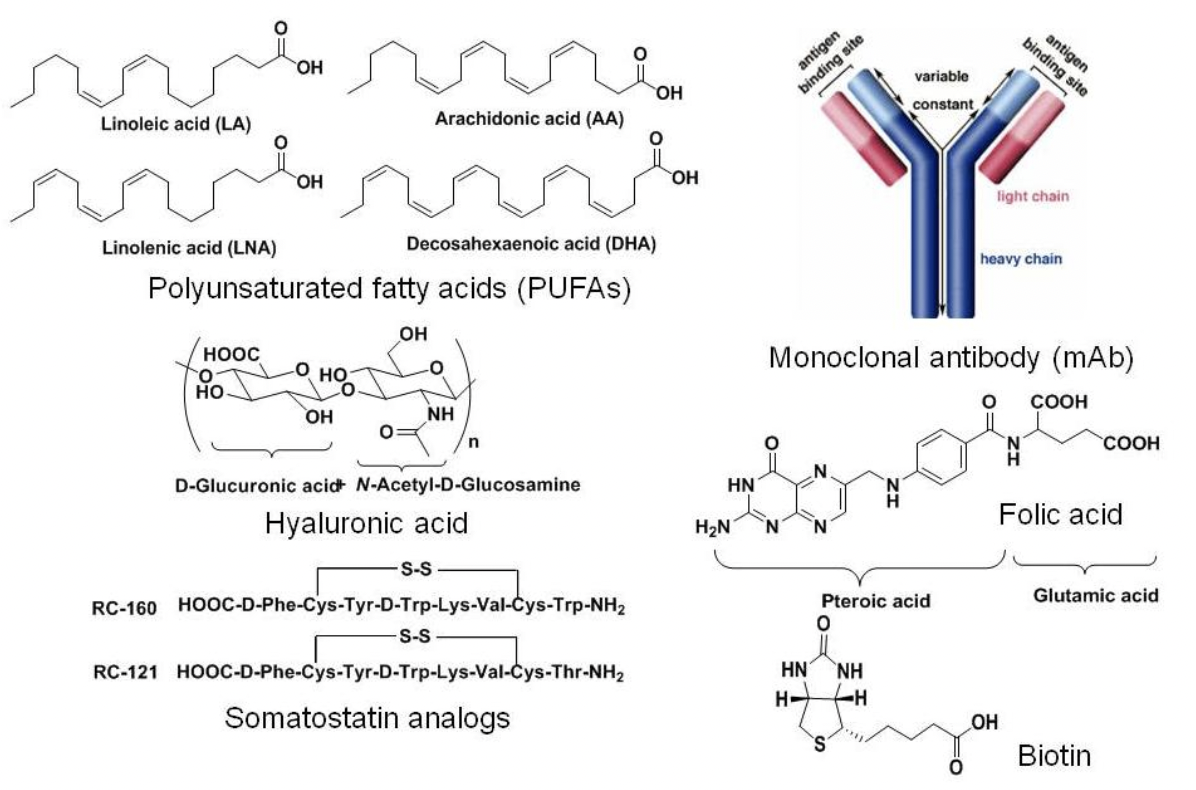
Tumor Targeting Molecules
S. Jaracz, J. Chen, L. V. Kuznetsova, and I. Ojima, "Recent Advances in Tumor-targeting Anticancer Drug Conjugates", Bioorg. Med. Chem., 13, 5043-5054 (2005).
Protein-Mediated TTM Internalization
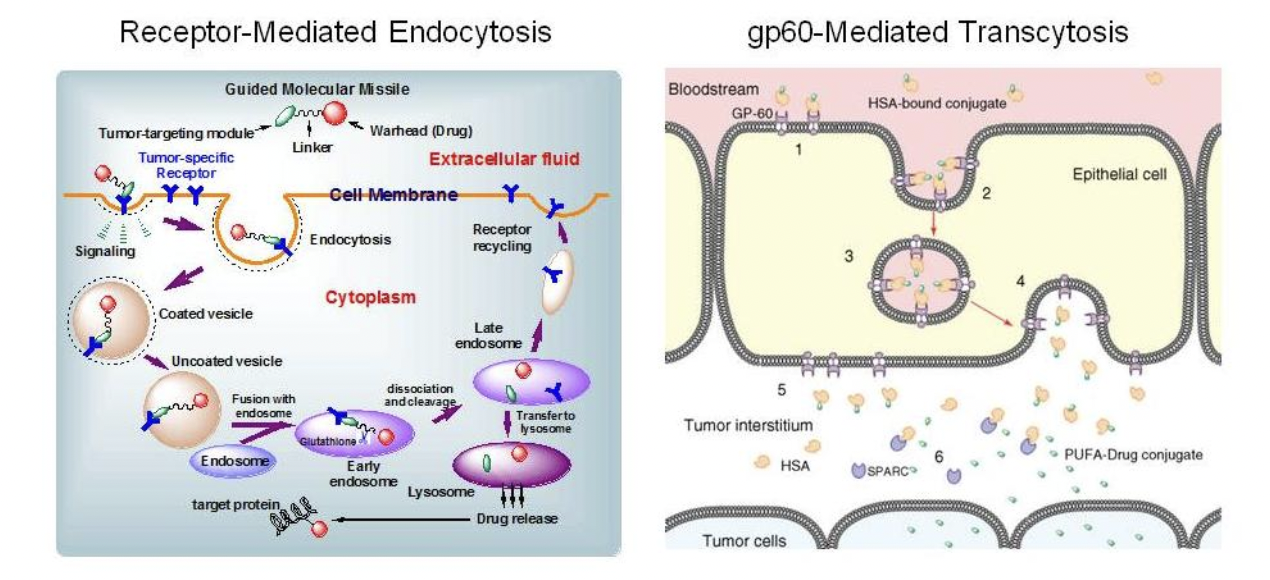
Ojima, I. "Guided Molecular Missiles for Tumor-Targeting Chemotherapy: Case Studies
Using the 2nd-Generation Taxoids as
Warheads", Acc. Chem. Res. 2008, 41, 108-119.
Chen, S., Zhao, X., Chen, J., Chen, J., Kuznetsova, L., Wong, S. S., & Ojima, I. "Mechanism-based tumor-targeting drug delivery system. Validation of efficient vitamin receptor-mediated endocytosis and drug release." Bioconjugate Chemistry 21, no. 5 (2010): 979-987.
Seitz, J. D., Ojima, I. Drug Delivery in Oncology: From Basic Research to Cancer Therapy. Ed. Kratz, F., et. al. In Press
First Generation mAb-Taxoid Drug Conjugate
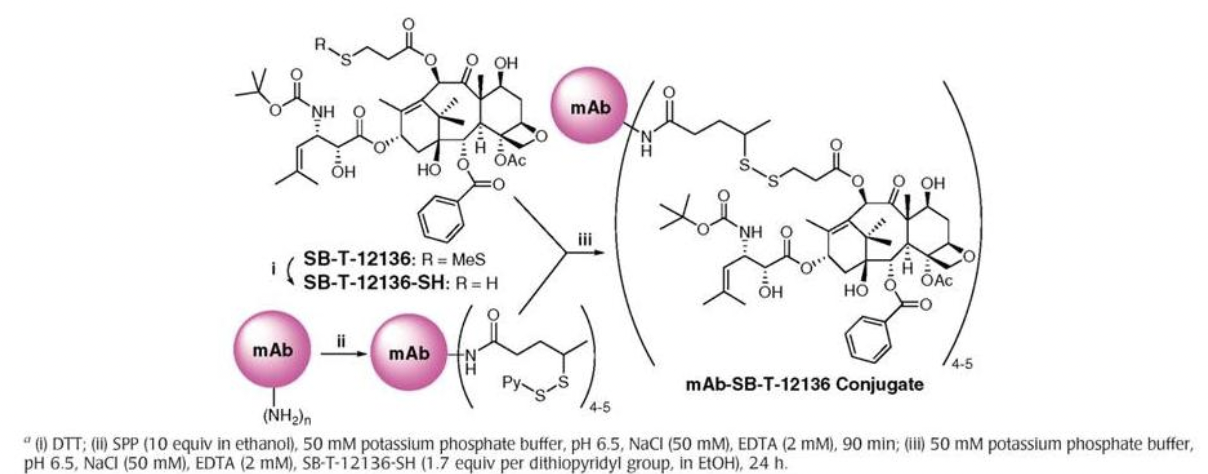
Ojima, I. "Guided Molecular Missiles for Tumor-Targeting Chemotherapy: Case Studies Using the 2nd-Generation Taxoids as Warheads", Acc. Chem. Res. 2008, 41, 108-119.
New Self-Immolative Linkers for Taxoid Conjugates
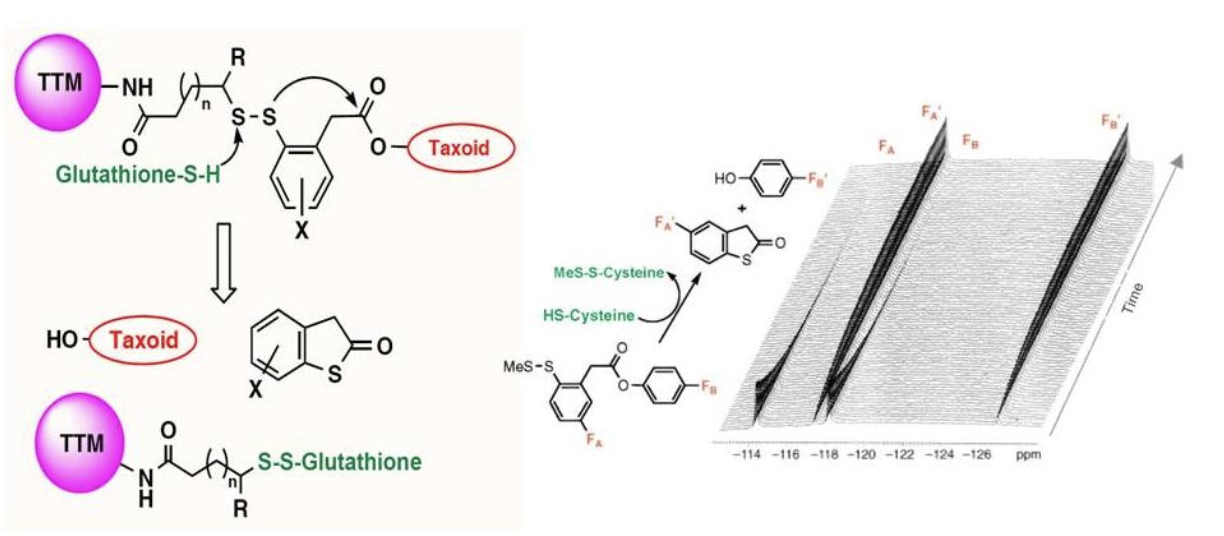
Ojima, I. "Use of fluorine in the medicinal chemistry and chemical biology of bioactive compounds—A case study on fluorinated taxane anticancer agents." Chem. Bio. Chem. 5, 628-635(2004).
Ojima, I. "Guided Molecular Missiles for Tumor-Targeting Chemotherapy: Case Studies Using the 2nd-Generation Taxoids as Warheads", Acc. Chem. Res. 2008, 41, 108-119.
Metabolic Stability Assessment of Tumor-Targeted Drug Delivery Systems by 19F NMR
Novel biotin-linker-taxoid conjugates were synthesized with strategically incorporated fluorine substituents to observe disulfide linker cleavage by 19F NMR. Ring substitution of fluorine on the linker and formulation with excipients significantly altered the rates of disulfide exchange and thiolactonization. BLT-S-F6, with improved solubility and heightened sensitivity, demonstrated strong stability in blood plasma, but readily cleaved and released the taxoid when exposed to glutathione levels found in the cytosol.
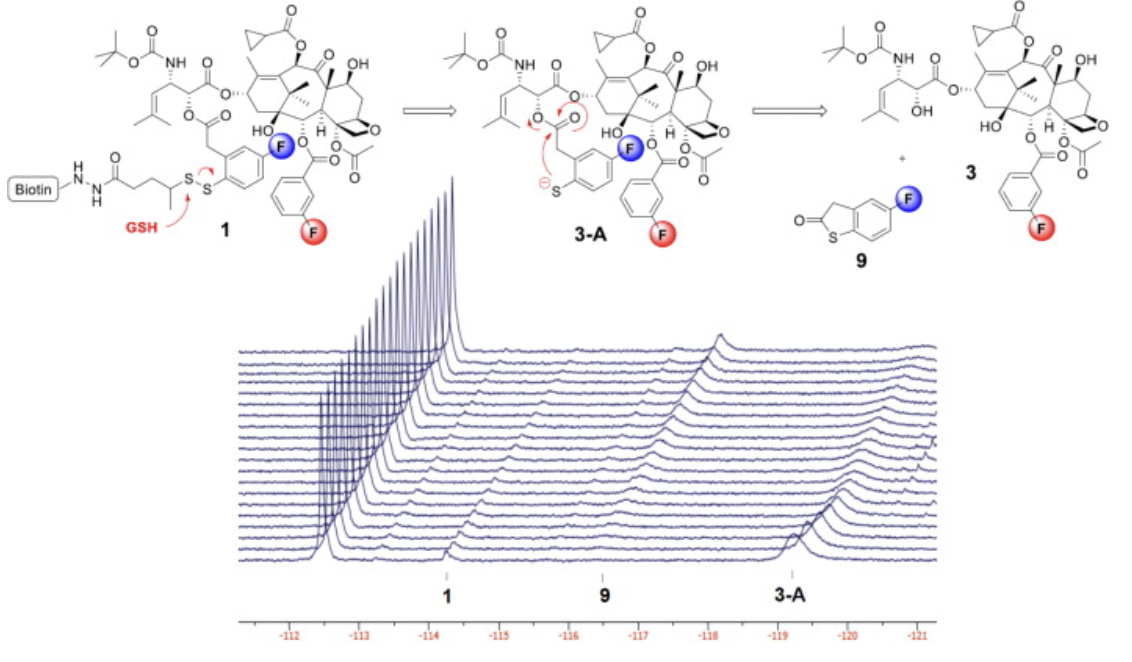
Time-resolved 19F NMR observation of thiolactonization of [2.5 mM] probe 1 in 30% DMSO in D2O beginning 1 hour after the addition of 6 equivalents of GSH at 25 °C at intervals of 15 min (128 scans/spectrum). This 19F NMR experiment disclosed that the "self-immolation" of the disulfide linker proceeded in two steps, generating the mechanistically anticipated thiolate 3-A as detectable transient species/intermediate, prior to thiolactonization to release a drug. It also suggests that the introduction of a fluorine para to a disulfide linkage had a profound effect on the rate of linker cleavage as well as thiolactonization.
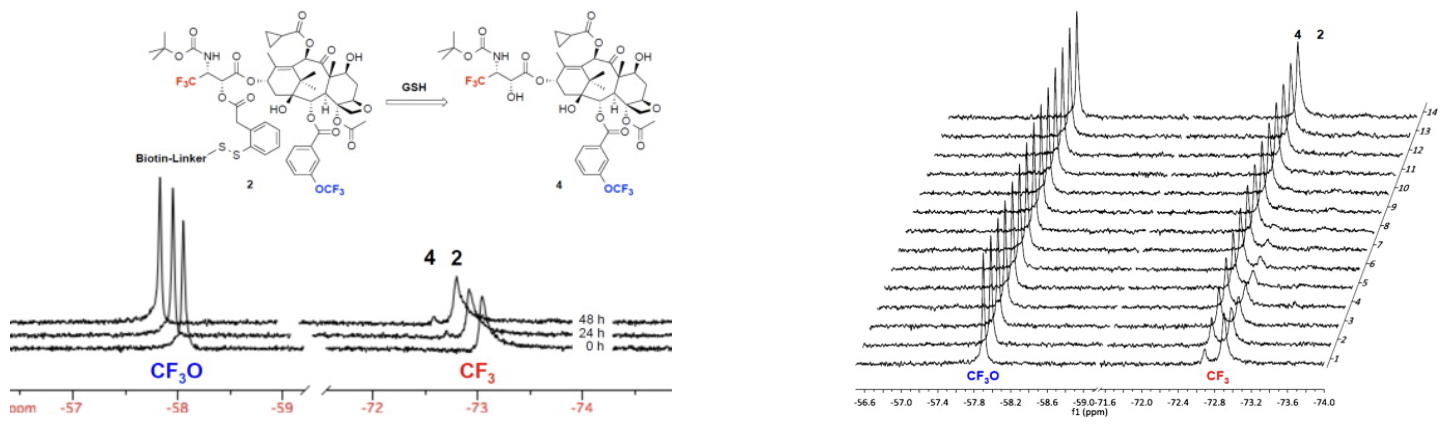
Left: 19F NMR spectral overlay of drug release of [200 μM] solution of BLT-S-F6 (2) in 86% blood plasma, 10% D2O, 2% ethanol, and 2% Tween 80 at 37 °C without supplemental GSH at 0, 24, and 48 h (1024 scans/spectrum). The result implies that the putative half-life of drug conjugates and TTDDSs using this self-immolative linker unit and taxoids as warheads in this formulation would be longer than one week in human blood plasma. Right: Time-resolved 19F NMR observation of drug release of [200 μM] BLT-S-F6 (2) in 86% blood plasma, 2% ethanol, and 2% Tween 80 in D2O 30 min after the addition of 100 equivalents of GSH at 37 °C at intervals of 1 h (1024 scans/spectrum). >98% of probe 2 disappeared and the corresponding amount of fluorotaxoid 4 appeared within 10 hour at 37 °C. The half-life of probe 2 with 100 equivalents of GSH in blood plasma was calculated to be ~3 h. This experiment allows us to estimate the half-life of this self-immolative disulfide linker in the cytosolic compartments following RME.
J. D. Seitz, J. G.Vineberg, L. Wei, J. F. Khan,B. Lichtenthal, C.-F. Lin and I. Ojima, "Design, synthesis and application of fluorine-labeled taxoids as 19F NMR probes for the metabolic stability assessment of tumor-targeted drug delivery systems" J. Fluor. Chem., 171 (2015), 148-161.
Fluorescence-Labeled Probes
Ojima, I. "Guided Molecular Missiles for Tumor-Targeting Chemotherapy: Case Studies
Using the 2nd-Generation Taxoids as
Warheads", Acc. Chem. Res. 2008, 41, 108-119.
Chen, S., Zhao, X., Chen, J., Chen, J., Kuznetsova, L., Wong, S. S., & Ojima, I. "Mechanism-based tumor-targeting drug delivery system. Validation of efficient vitamin receptor-mediated endocytosis and drug release." Bioconjugate Chemistry 21, no. 5 (2010): 979-987.
Theranostic Vitamin−Linker−Taxoid Conjugates
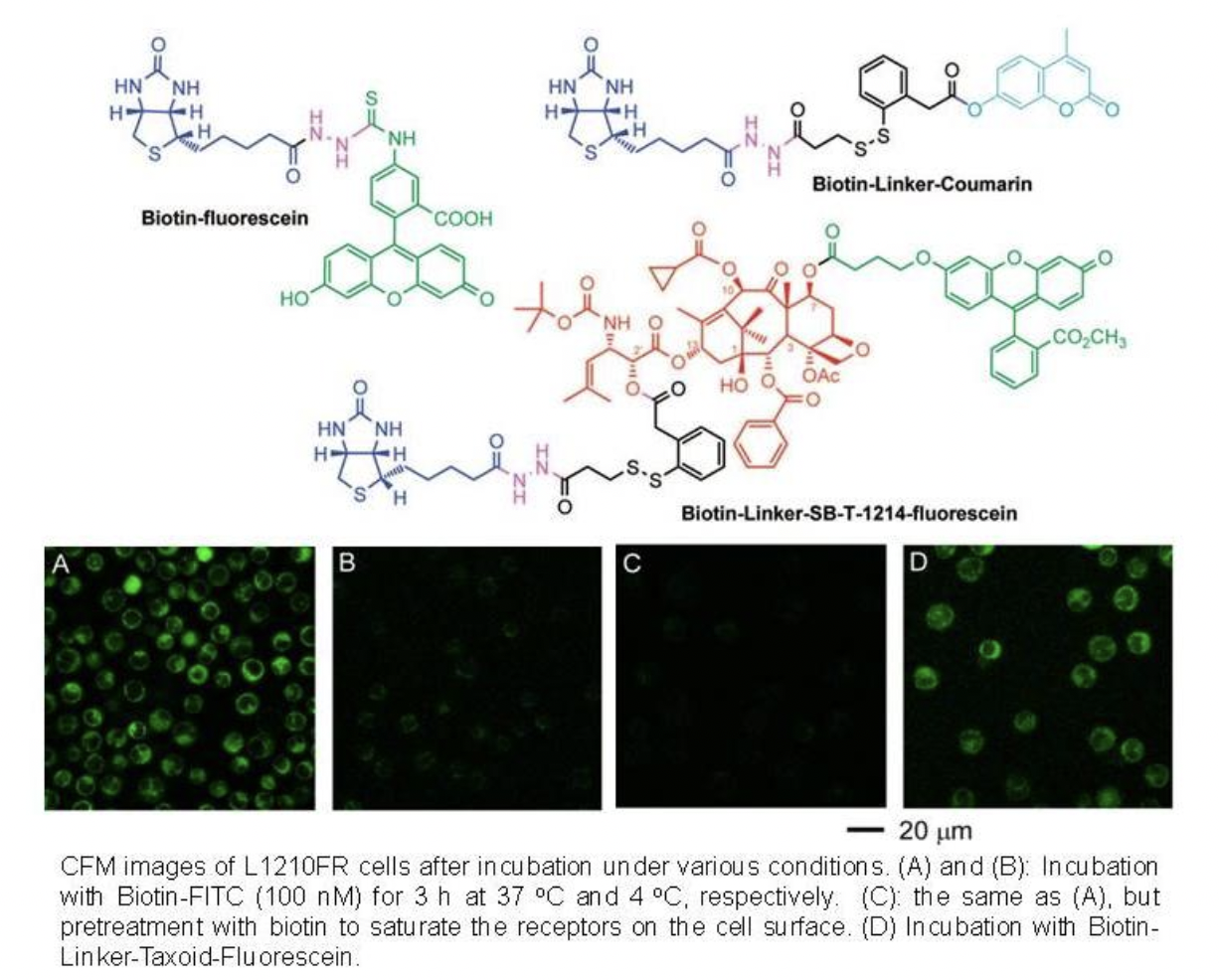
Recently, we have designed and synthesized novel tumor-targeting theranostic conjugates 1 and 2, which consist of biotin as the tumor-targeting module, SB-T-1214 as the cytotoxic agent, a selfimmolative disulfide linker for drug release, 1,3,5-triazine as the splitter module, ethylene glycol oligomers to increase aqueous solubility, and either a fluorine-labeled prosthetic in 1 for potential 18F-PET imaging in vivo or an FITC tether in 2 for internalization and drug-release studies in vitro. This TTDDS exhibited very high specificity to BR+ cancer cells.
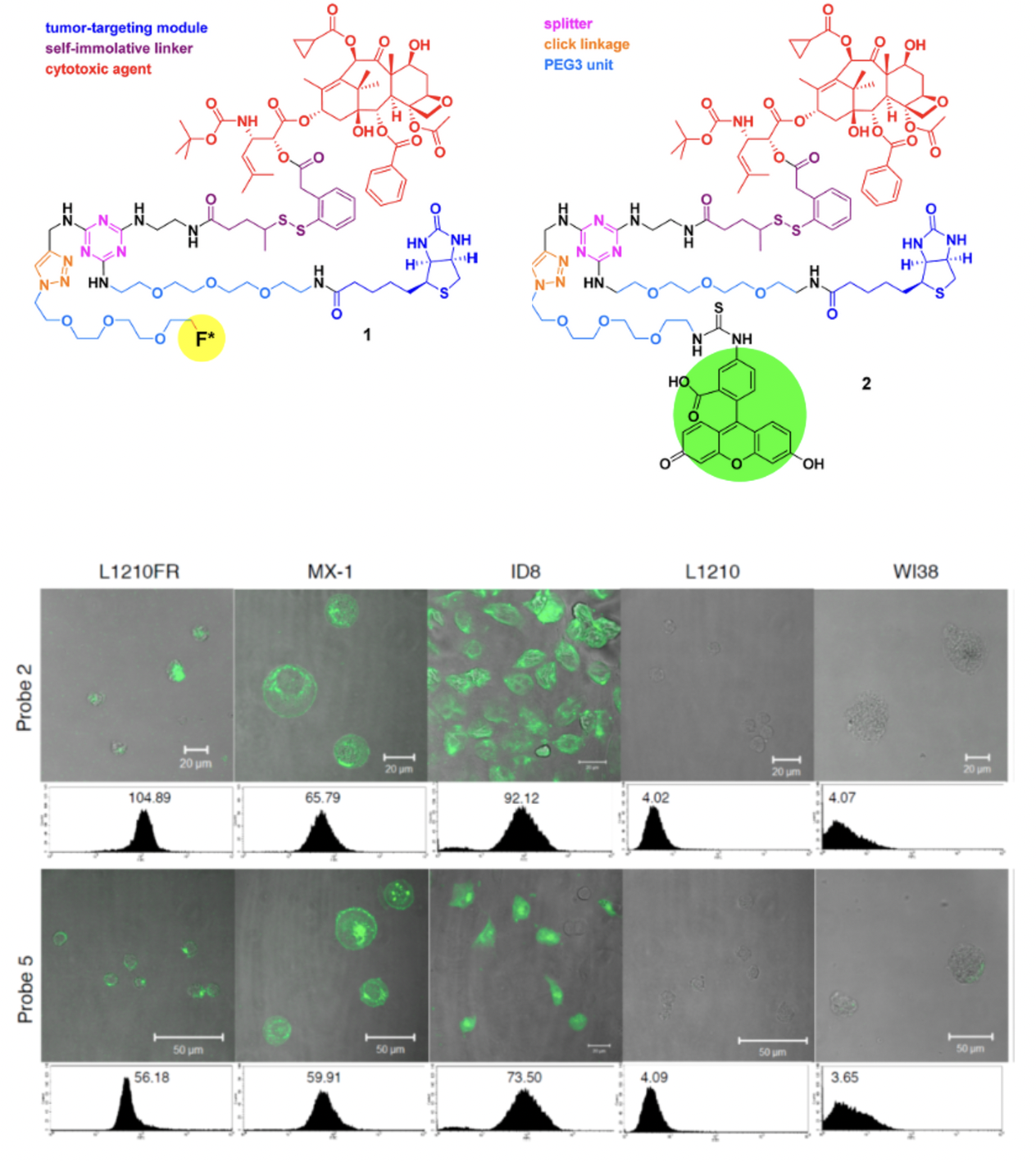
CFM images and flow cytometry analysis of L1210FR (BR+), MX-1 (BR+), ID8 (BR+), L1210 (BR−), and WI38 (BR−) cell lines after incubation with conjugate 2 (5 uM) (upper row) or probe 5 (5 uM) (lower row) at 37ºC for 3 h.
Vineberg, G., Wang, T., Zuniga, E., Ojima, I., Design, Synthesis, and Biological Evaluation of Theranostic Vitamin− Linker−Taxoid Conjugates, J. Med. Chem., ASAP 2015.
Drug Release
Ojima, I. "Guided Molecular Missiles for Tumor-Targeting Chemotherapy: Case Studies Using the 2nd-Generation Taxoids as Warheads", Acc. Chem. Res. 2008, 41, 108-119.
Chen, S., Zhao, X., Chen, J., Chen, J., Kuznetsova, L., Wong, S. S., & Ojima, I. "Mechanism-based tumor-targeting drug delivery system. Validation of efficient vitamin receptor-mediated endocytosis and drug release." Bioconjugate chemistry 21, no. 5 (2010): 979-987.
Internalization and Drug Release of Biotin-CNT-Linker-Taxoid-Fluorescein
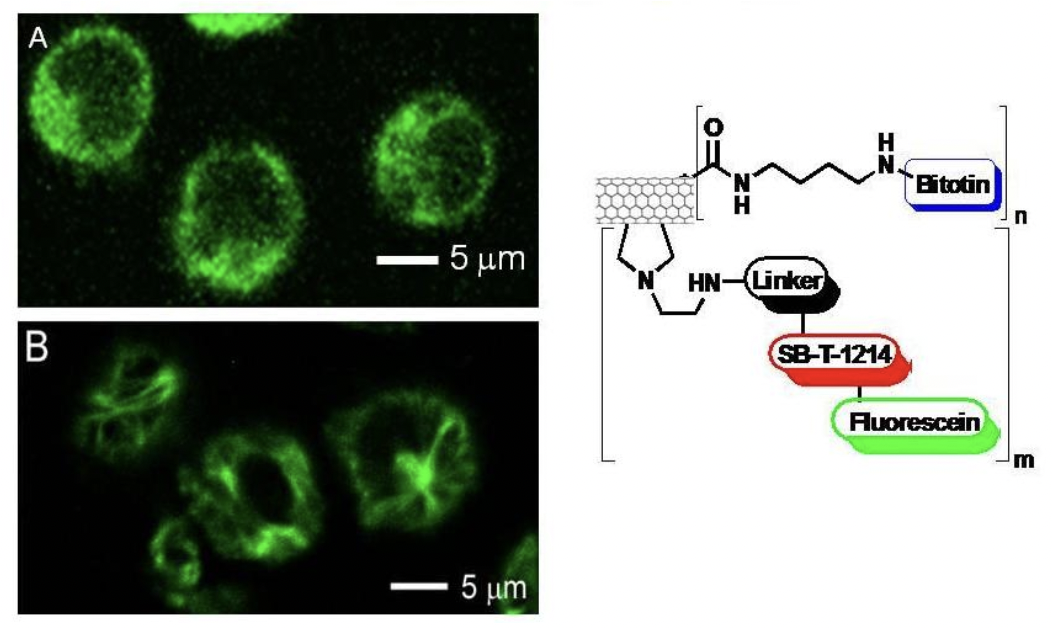
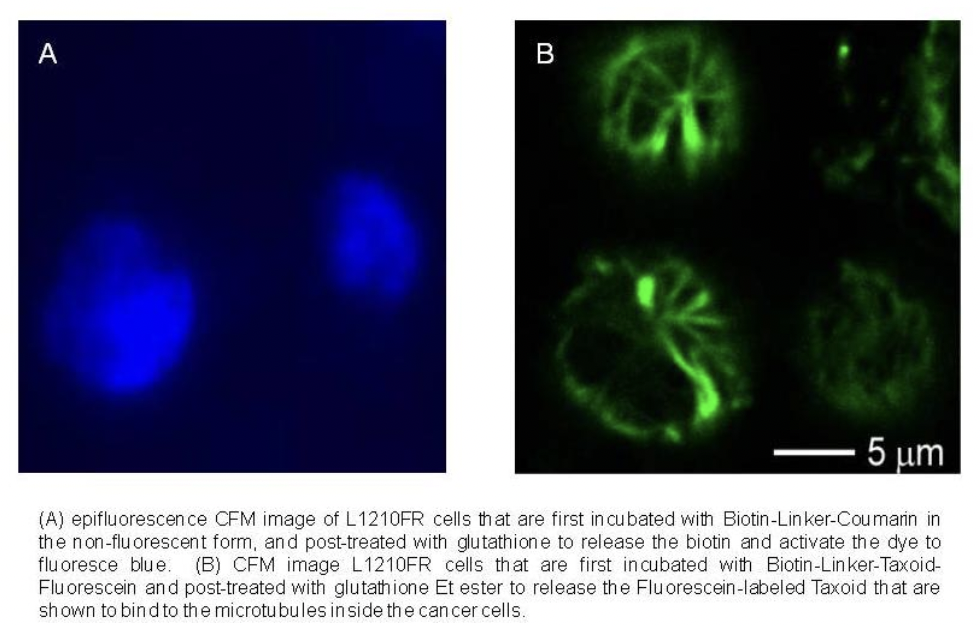
CFM images of L1210FR cells treated with biotin-CNT-taxoid-fluorescein incubated in the absence (A) and in the presence (B) of GSH Et ester. The latter shows the presence of a microtubule network, polymerized by taxoid, after the disulfide bonds had been cleaved by the GSH ethyl ester.
Advantages of CNT-based DDS:
- Amplification of tumor-targeting drug delivery via the “enhanced permeability and retention” (EPR) effect associated with nano-scale materials.
- Ability to exploit multivalent recognition of overexpressed receptors on the surfaces of the tumor cells.
- Increased drug loading capacity
- Capability of controlled drug release inside tumor cells.
J. Chen S. Chen, X. Zhao, L. Kuznetsova, S. S. Wong, I. Ojima, Functionalized single-walled carbon nanotubes as rationally designed vehicles for tumor-targeted drug delivery. J. Am. Chem. Soc. 130, 16778-16785 (2008).
Asymmetric Bow-Tie Dendrimer-based (ABTD) Drug Delivery System
Dendrimer is a class of tree-like polymer with well-defined and shaped structure and good biocompatibility. It has been used as a macromolecular carrier to deliver anticancer drugs in tumor-targeted drug delivery. However, traditional dendrimer-based drug delivery systems directly decorate a dendrimer with different functionalities resulting in a mixture unable to be isolated to single pure components. To solve this problem, an advanced strategy is designed in Ojima Group by fully functionalizing different generations PAMAM dendrimer bearing cleavable cystamine core, followed by the cleavage of the fully functionalized dendrimers to generate two half dendrons. Then the two half dendrons could be re-coupled through a suitable linker to construct an ABTD versatile platform, which can be converted to a series of conjugates through an orthogonal click reaction. Each intermediate can be isolated as a single component with determined molecular weight. This ABTD system is a combination of active and passive targeting strategies. The tumor-targeting moiety (TTM) is recognized by its receptor overexpressed on the surface of cancer cells, and multiple TTMs can possess multi-binding effect to enhance the receptor-mediated endocytosis (RME) process for active targeting. Passive targeting replies on enhanced permeability and retention (EPR) effect which is typical for nano-sized particle. In general, this ABTD drug delivery system consists of sixteen polyethylene glycolylated (PEGylated) TTM on generation 3 half dendron, coupling through a PEGylated bis(maleicimido) linker to generation 1 half dendron with four PEGylated cytotoxic warheads each with/without a fluorescent probe and connected through a self-immolative smart disulfide linker. Excellent progress have been made to prove ABTD system is highly efficient for delivering anticancer drugs in tumor-targeted drug delivery.
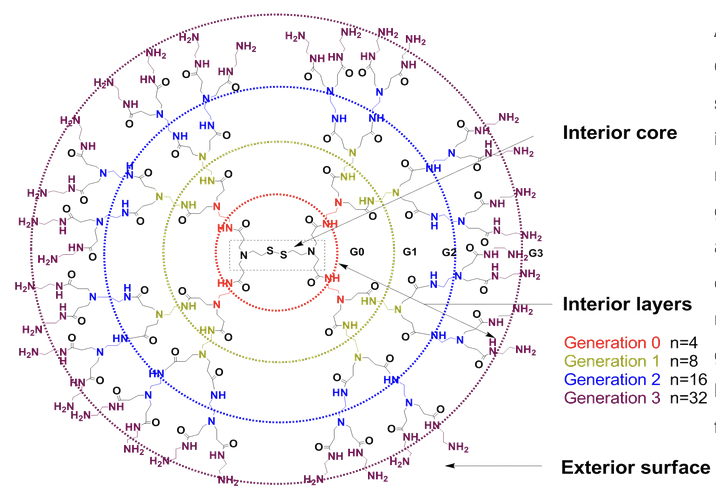
Architecture of PAMAM (poly(amidoamine)) Dendrimer with Cleavable Cystamine Core: PAMAM (poly(amidoamine)) dendrimer has well-defined structure. Its tree-like molecular architecture can be divided to an interior core, interior layers (also called "generations") which consists of repeating units regularly attached to the core, and an exterior surface of functional terminal groups attached to the outermost generation. Generation number (usually abbreviated to G#) is determined by the number of focal points going from the core towards the surface. The molecular weight of dendrimer itself and the number of terminal functional group can be easily determined by the generation. Especially the dendrier with a cleavable cystamine core (disulfide linkage) can be homo-cleaved to form two half dendrons with sulfhydryl functional group.
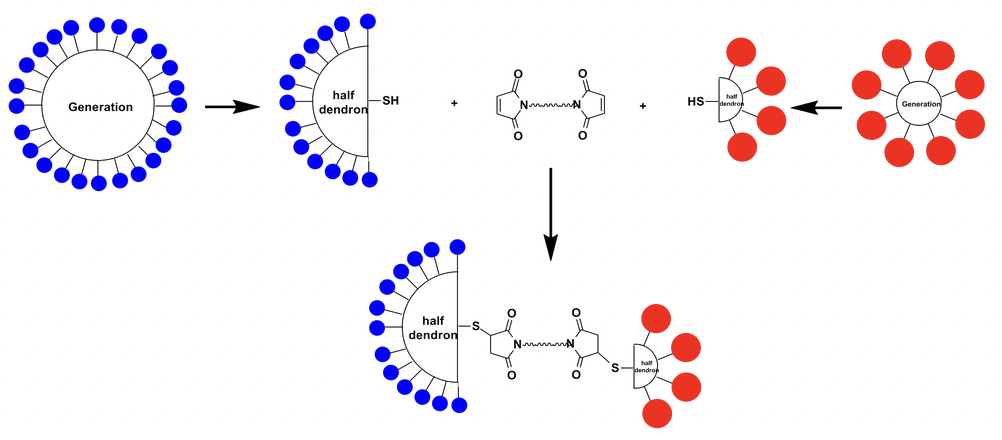
Synthetic Strategy for Construction of Asymmetric Bow-Tie Dendrimer (ABTD) Platform: Instead of attaching different functionalities on one dendrimer molecule resulting in a mixture, in Ojima group we developed the synthetic strategy to generate dendrimer-based conjugate as determined and repeatable single component macromolecule. Different generations of dendrimer bearing cleavable core are fully functionalized by one type but different functionality, and cleaved to form half dendrons, followed by re-coupled through a suitable linker to construct a dendrimer-based platform. And we have developed purification method for isolating each macromolecular intermediate as single component with good purity.
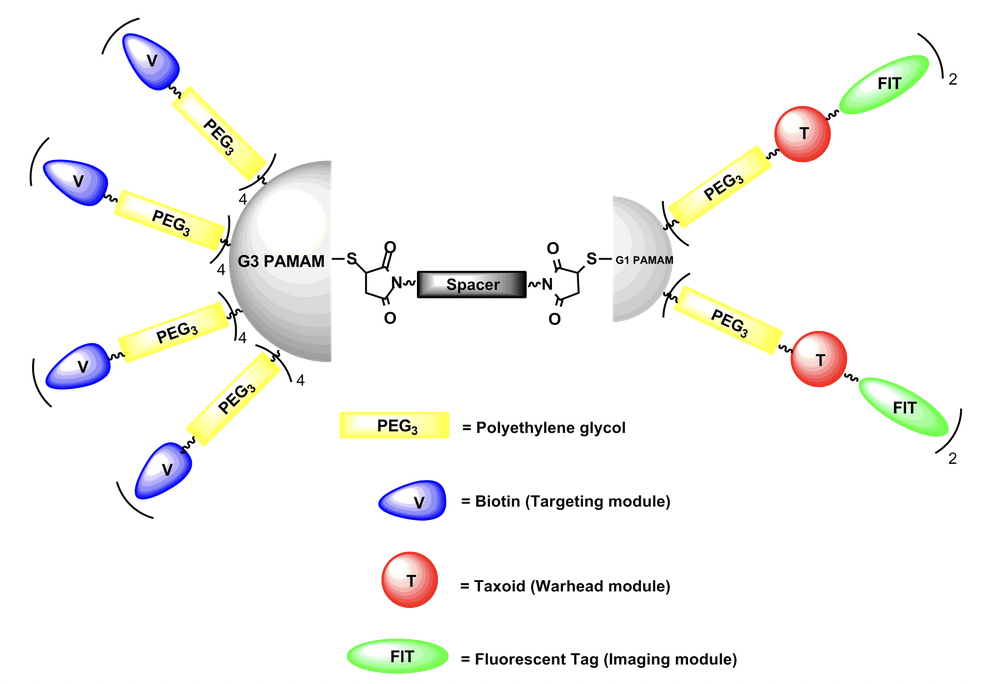
Model Structure of Asymmetric Bow-Tie Dendrimer (ABTD) Conjugates: A versatile asymmetric bow-tie dendrimer (ABTD) platform is designed and a series of ABTD conjugates has been successfully synthesized. A PEGylated biotin (tumor-targeting moiety in blue) is functionalized on each arm of half dendron of generation 3 dendrimer, a PEGylated linker-taxoid (warhead in red) with or without fluorescent probe (imaging probe in green) is functionalized on each arm of half dendron of generation 1 dendrimer, and connected through a PEGylated bis(maleimido) linker to afford a novel nano-sized drug delivery system. This dendrimer-based system has shown a multi-binding effect due to the multiple tumor-targeting modules and high efficiency for delivering warhead in tumor-targeted drug delivery.
Design and synthesis of tumor-targeting theranostic drug conjugates for SPECT and PET imaging studies, Tao Wang,Jacob G. Vineberg, Tadashi Hondaand Iwao Ojima, Bioorg. Chem. 76, 458-467 (2018). PMID: 29287255
Design, Synthesis and Biological Evaluations of Asymmetric Bow-Tie PAMAM Dendrimer-based Conjugates for Tumor-Targeted Drug Delivery, Tao Wang,Yaozhong Zhang, Longfei Wei, Yuhan G. Teng,Tadashi Honda and Iwao Ojima, ACS Omega, 3(4), 3717–3736 (2018).PMC5928494
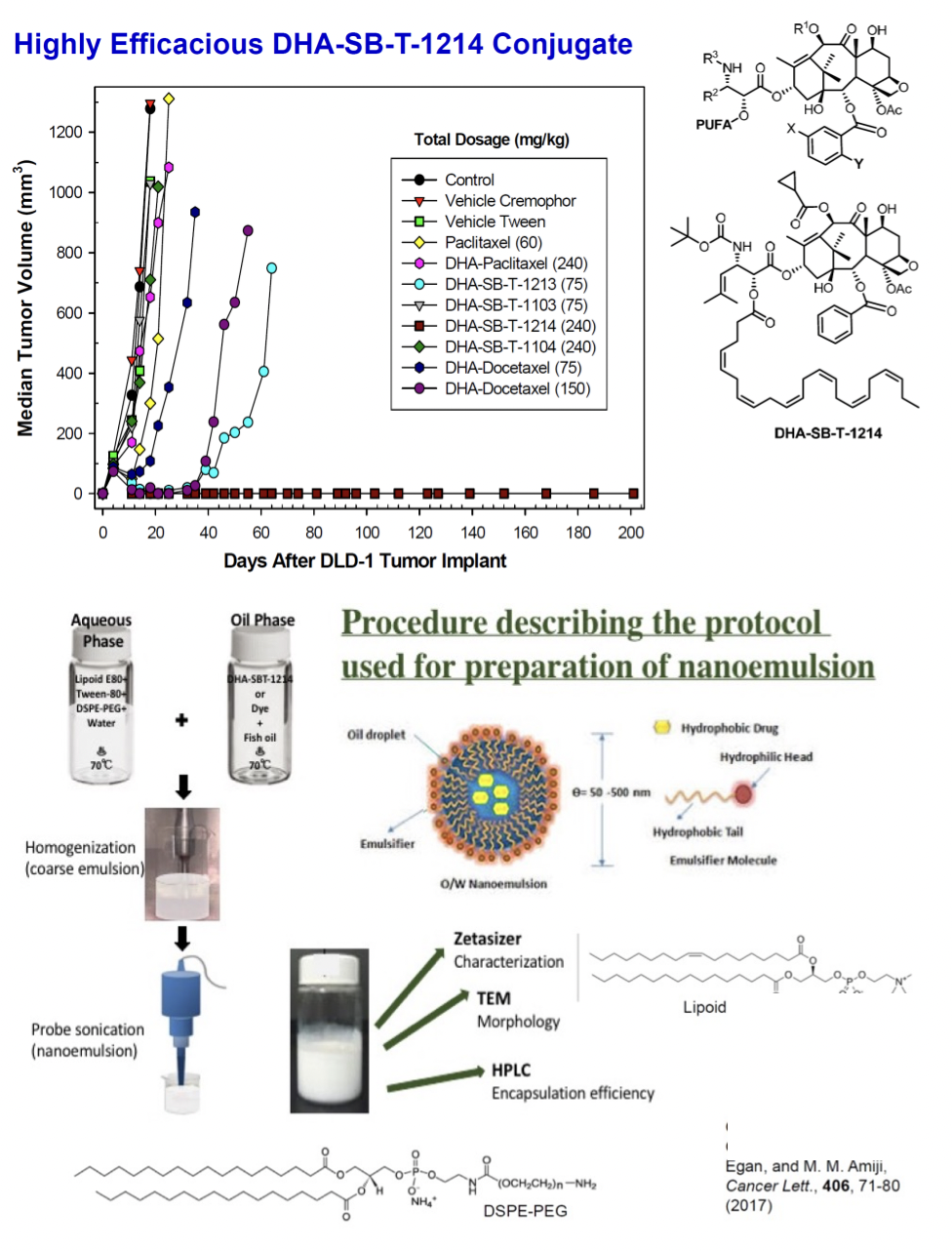
Nanoemulsion Formulation of a Novel Taxoid Prodrug SBT-1214 Conjugated with Omega-3 Fatty Acid Inhibits Prostate Cancer Stem Cell-Induced Tumor Growth, G. Ahmad, R. El-Sadda, G. Botchkina, I. Ojima, J. Egan, and M. M. Amiji, Cancer Lett., 406, 71-80 (2017). PMC5591776
High Capacity Poly(2-oxazoline) Micelles for 3rd Generation Taxoids: preparation, in vitro and in vivo evaluation, Z. He, A. Schulz, X. Wan, J. Seitz, H. Bludau, D. B. Darr, C. M. Perou, R. Jordan, I. Ojima, A. V. Kabanov, R. Luxenhofer, J. Control. Release 208, 67-75 (2015). PMID: 25725361
Nanoemulsion Formulation of a Novel Fatty Acid – Taxoid Conjugate: NE-DHA-SBT-1214
Nanoemulsions are dispersions of two different liquids that are usually immiscible with each other. Depending on the order of adding the liquids, these are either called water-in-oil or oil-in-water and are nanometers in size. Nanoemulsion formulations are the most commonly used carriers for hydrophobic drug delivery due to their effective therapeutic ability both in vitro and in vivo. Many anticancer drug-encapsulated nanoemulsions have shown enhanced efficacy due to their target-specific systemic delivery to tumor sites and EPR effect.
NE-DHA-SBT-1214 nanoemulsions were characterized and compared with solution formulations in PPT2 patient-derived prostate cancer stem cells (CSCs), demonstrating superior in vitro delivery efficiency and cytotoxicity. In vivo studies showed that NE-DHA-SBT-1214 had comparative efficacy to Abraxane and placebo nanoemulsions, influencing the clonogenic and sphere-forming capabilities of tumor cells. Confocal imaging revealed significant intracellular uptake of dye-encapsulated NEs in both monolayer and spheroid PPT2 cultures.
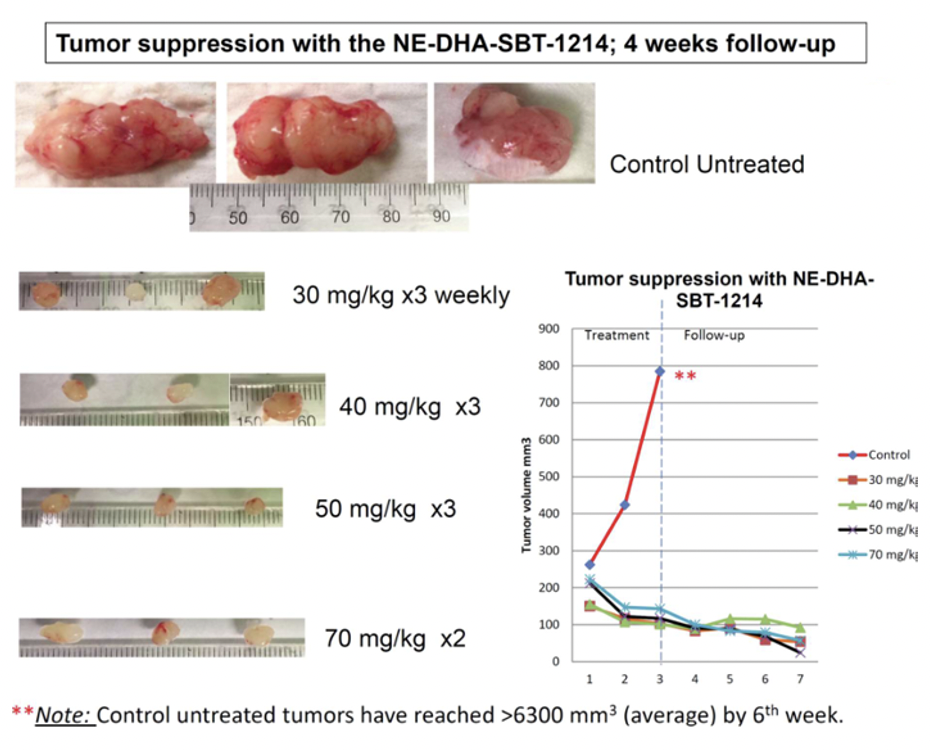
After PPT2 cell transplantation, NOD/SCID mice received weekly intravenous treatments with NE-DHA-SBT-1214 (25, 30, 40, 50, 70 mg/kg), Abraxane (25, 40 mg/kg), or vehicle, with four mice per group (six for the 25 mg/kg NE-DHA-SBT-1214 group). NE-DHA-SBT-1214 significantly suppressed tumor growth, surpassing Abraxane™, with dose-dependent tumor volume reductions of 45% (25 mg/kg), 62% (30 mg/kg), 74% (40 mg/kg), and 88% (50 mg/kg) compared to controls.
NE-DHA-SBT-1214 treatment significantly reduced the clonogenic potential of CSC-enriched PPT2 tumor xenograft cells, resulting in small, capillary-lacking tumors that failed to form holoclones or spheroids. Post-treatment, viable cells could not induce floating spheroids or holoclone formation, indicating substantial cell death.
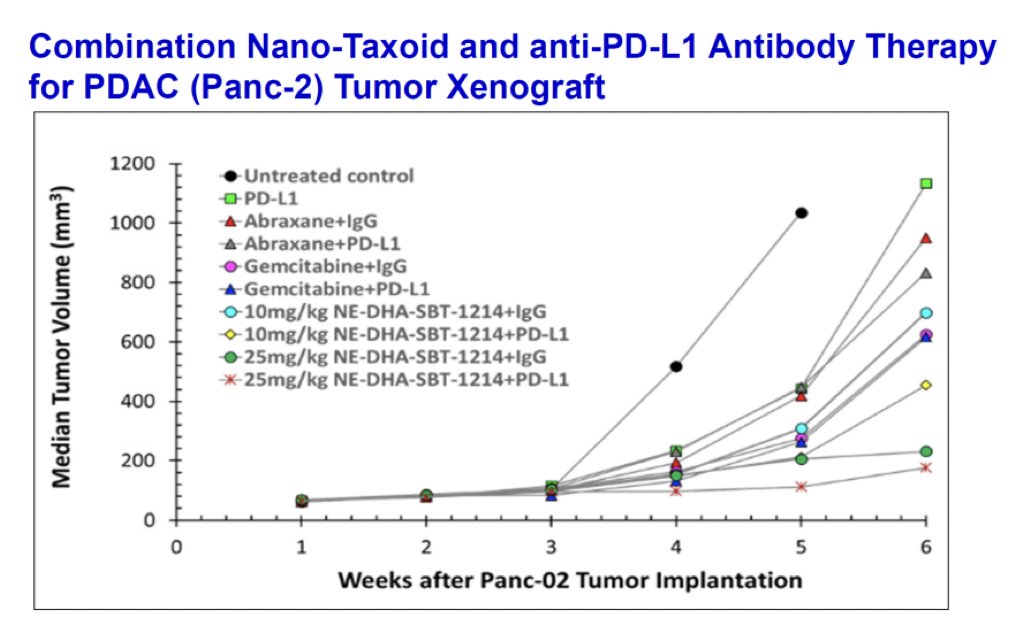
Combination of NE-DHA-SBT-1214 with Cancer Immunotherapy
In vivo anti-tumor efficacy evaluation based on changes in subcutaneous syngeneic Panc-2 tumor volume in C57BL/6 mice upon treatment with gemcitabine, Abraxane, or NE-DHA-SBT-1214 alone or in combination with an anti-PD-L1 antibody. The intravenous doses of NE-DHA-SBT-1214 were 10 mg/kg and 25 mg/kg and the anti-PD-L1 antibody was dosed at 200 µg per dose, three doses per week. A combination of anti–PD-L1 therapy with NE-DHA-SBT-1214 significantly enhanced CD8+ T-cell infiltration and significant tumor growth suppression, clearly surpassing Abraxane.
Related Publications
1. Kuznetsova, L.; Chen, J.; Sun, L.; X. Ben Wu; Pepe, A.; Veith, J.; Pera, P.; Bernacki, R. J.; Ojima, I., Syntheses and Evaluation of Novel Fatty Acid-Second-Generation Taxoid Conjugates as Promising Anticancer Agents. Bioorganic & Medicinal Chemistry Letters2006, 16 (4), 974–977.
2. Ahmad, G.; El Sadda, R.; Botchkina, G.; Ojima, I.; Egan, J.; Amiji, M., Nanoemulsion Formulation of a Novel Taxoid DHA-SBT-1214 Inhibits Prostate Cancer Stem Cell-Induced Tumor Growth. Cancer Letters2017, 406, 71–80.
3. Ahmad, G.; Mackenzie, G. G.; Egan, J.; Amiji, M. M., DHA-SBT-1214 Taxoid Nanoemulsion and Anti–PD-L1 Antibody Combination Therapy Enhances Antitumor Efficacy in a Syngeneic Pancreatic Adenocarcinoma Model. Molecular Cancer Therapeutics2019, 18 (11), 1961–1972.
4. Eagan, J. E.; Amiji, M. M.; Ojima, I., Combination Taxoid Nanoemulsion with Immunotherapy in Cancer, US Patent11,497,713 B2 (11/15/2022).
Poly(2-oxazoline)-based Micelles Formulation of 3rd-generation Taxoids
A novel polymeric drug carrier system utilizing block copolymers of hydrophilic poly(2-methyl-2-oxazoline) (PMeOx) and mildly hydrophobic poly(2-butyl-2-oxazoline) (PBuOx) has been developed. Despite the low hydrophobicity of PBuOx, these block copolymers, particularly triblock copolymers, demonstrate exceptional efficacy in solubilizing highly hydrophobic drugs like taxanes. POx/PTX micelles achieve approximately 4 to 5 times higher drug loading and 10 to 20 times higher drug concentrations in injectable formulations compared to Taxol, Genexol-PM, and Abraxane.
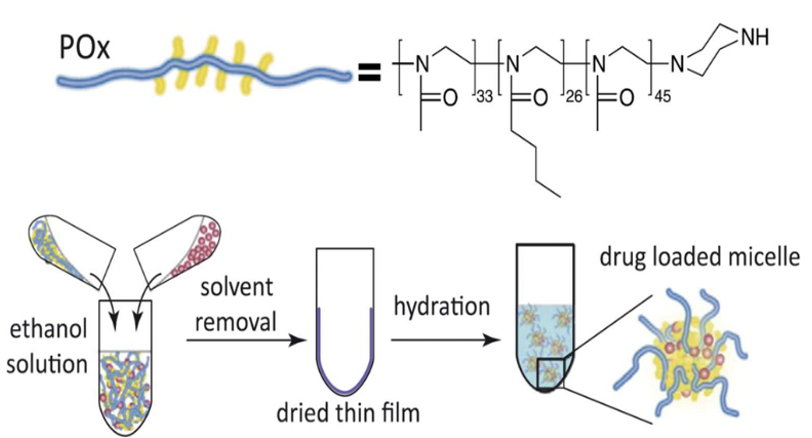
In vitro cytotoxicity of POx/SB-T-1214 micelles in cancer cells
In multidrug-resistant LCC6-MDR cells, the cytotoxicity profile of POx/SB-T-1214 clearly shifted to lower concentrations compared to the other three formulations of PTX. MTT assays in LCC6-WT cells observed IC50 values of the same order of magnitude for all four formulations.
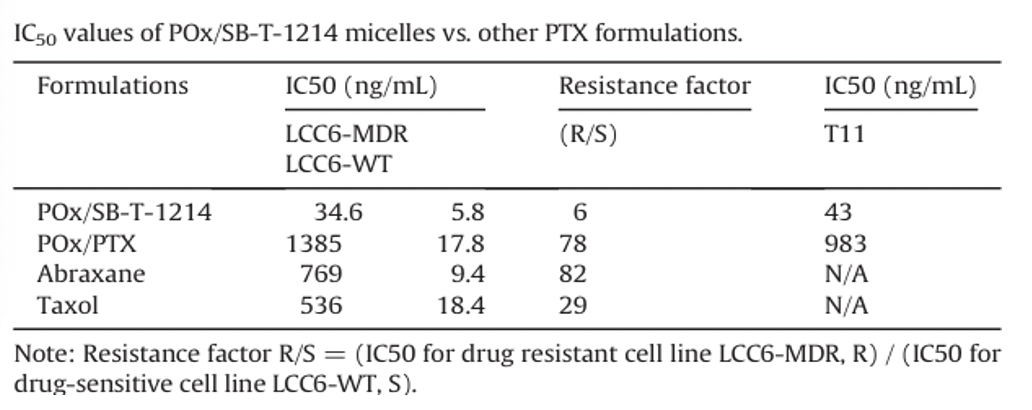
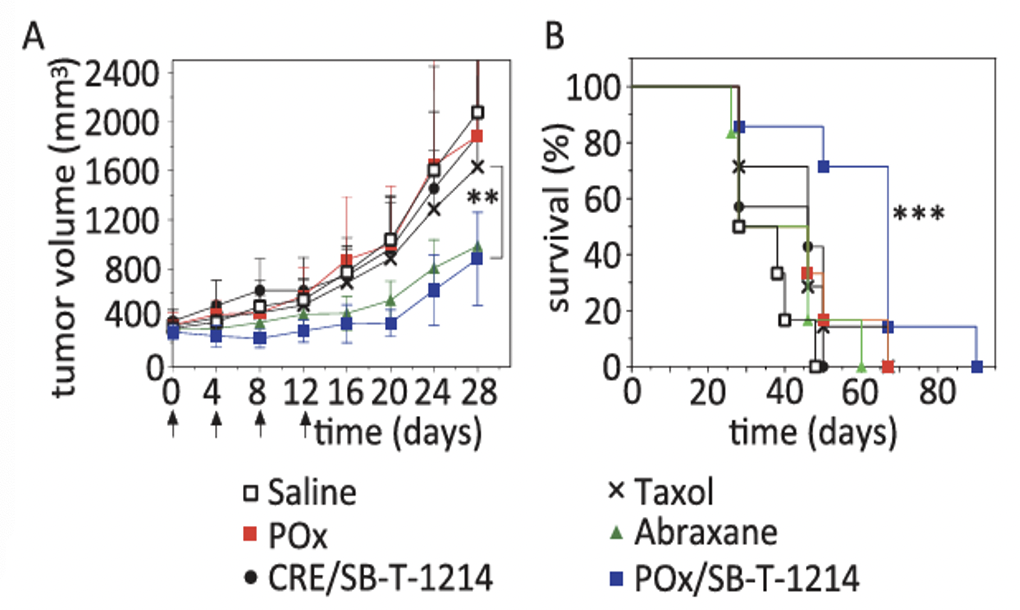
In vivo efficacy of POx/SB-T-1214 micelles in the LCC6-MDR model
Tumor growth inhibition of POx/SB-T-1214 (50/40 formulation, 20 mg/kg) was compared to Taxol (20 mg/kg), Abraxane (80 mg/kg), CRE/SB-T-1214 (20 mg/kg), saline and POx polymer alone. Each formulation was injected on days 0, 4, 8 and 12. While the tumor growth for the Abraxane-treated group was similar to that in the POx/SB-T-1214 group, no statistically significant difference in survival was observed between the Abraxane and saline groups. In contrast, treatment with POx/SB-T-1214 significantly extended the survival time, with a median survival of 67 days (p = 0.0003).
Related Publications
1. He, Z.; Schulz, A.; Wan, X.; Seitz, J.; Bludau, H.; Alakhova, D. Y.; Darr, D. B.; Perou, C. M.; Jordan, R.; Ojima, I.; Kabanov, A. V.; Luxenhofer, R. Poly(2-Oxazoline) Based Micelles with High Capacity for 3rd Generation Taxoids: Preparation, in Vitro and in Vivo Evaluation. Journal of Controlled Release2015, 208, 67–75.
Combination of EPR Effect and RME for Tumor-Targeted Drug Delivery of Taxoids
Low-Density Lipoprotein Receptor (LDLR) as a Novel Target for Tumor-Targeted Drug Delivery
LDL receptors, crucial for cholesterol metabolism, are often upregulated in cancer cells due to their high cholesterol demand. This makes LDL receptors a promising target for tumor-specific drug delivery. Encapsulating taxoids as LDL-mimicking nanoparticles can enhance their pharmacokinetics, biodistribution, and tumor-targeting specificity, addressing the issues of poor specificity, bioavailability, and toxicity associated with taxoids. The drug delivery system is engineered to (i) target LDL receptors overexpressed on tumor cells, (ii) facilitate efficient internalization via receptor-mediated endocytosis (RME), and (iii) ensure smooth drug release through hydrolysis of the carbonate linker. To monitor and validate these processes, fluorescent and fluorogenic molecular probes were designed and synthesized.
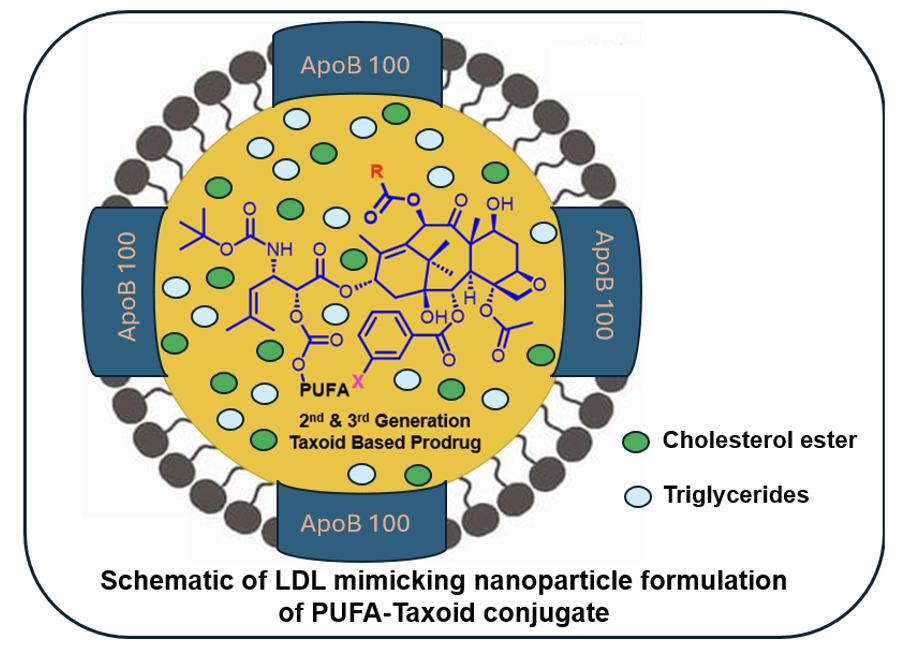
Proof of concept for LDLR-targeted drug delivery and drug release
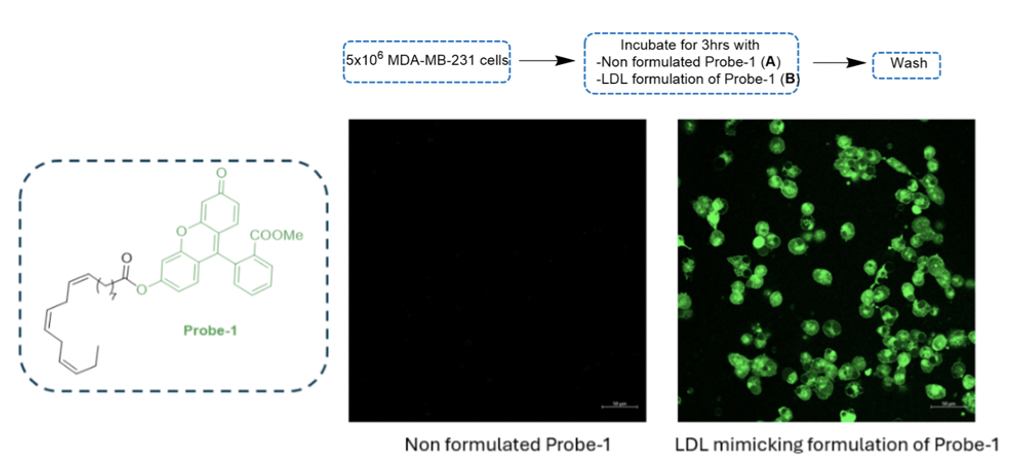
a) Receptor-Mediated Endocytosis (RME) of LDL-mimicking nanoparticles containing a fluorescent probe
To demonstrate internalization via receptor-mediated endocytosis (RME), a fluorescent probe-1 bearing fluorescein was synthesized. After incubating MDA-MB-231 cells with both unformulated probe-1 and LDL-formulated probe-1 for 3 hours, fluorescence was observed only with the LDL formulation, indicating successful RME internalization.
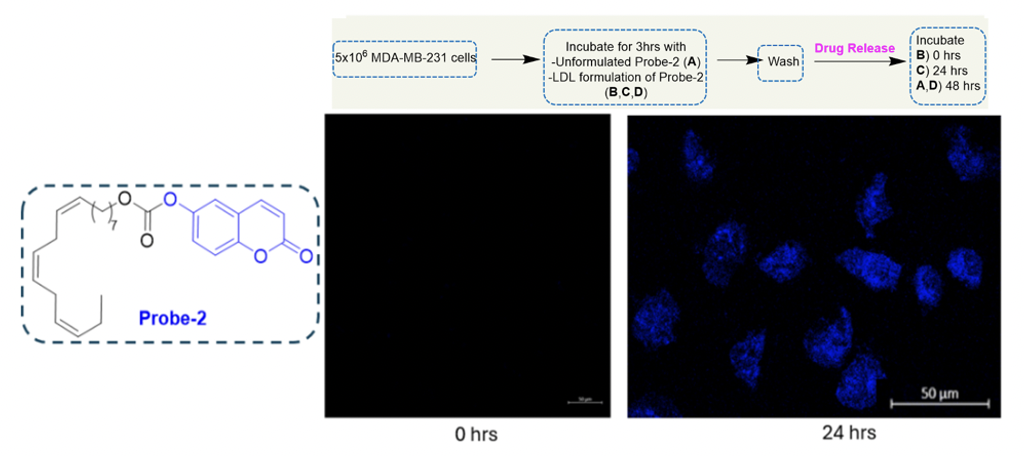
b) Drug release, following internalization via RME
A fluorogenic probe-2 was designed to investigate the release of the cytotoxic payload post-successful internalization via RME. CFM imaging was conducted at an incubation time of 0 hours and 24 hours. No fluorescence was observed at 0 hours; however, after 24 hours of incubation with the LDL formulation of probe-2, blue fluorescence was observed, indicating successful release of the cytotoxic payload post-RME.
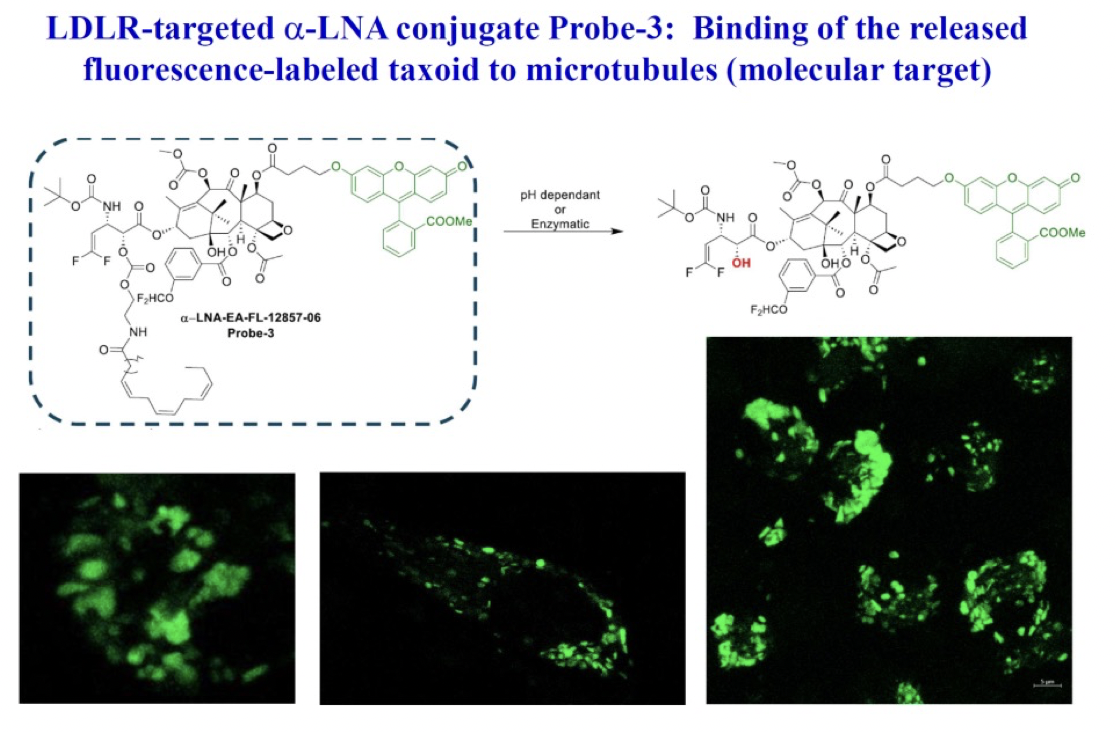
c) Target-binding of fluorescently labeled taxoid, following internalization via RME and drug release
To demonstrate the binding of the released warhead to microtubules post-internalization via RME, a C-7 fluorescein-labeled probe-3 was designed. Subsequent to a 72-hour incubation period of the LDL-mimicking formulation of probe-3 with the MDA-MB-231 cell line expressing LDL-R, CFM imaging was conducted. Following this, a Z-section experiment using CFM was performed to highlight the fluorescence-labeled microtubule bundles, conclusively indicating the successful binding of the released fluorescein-labeled taxoid to its molecular target (microtubules).
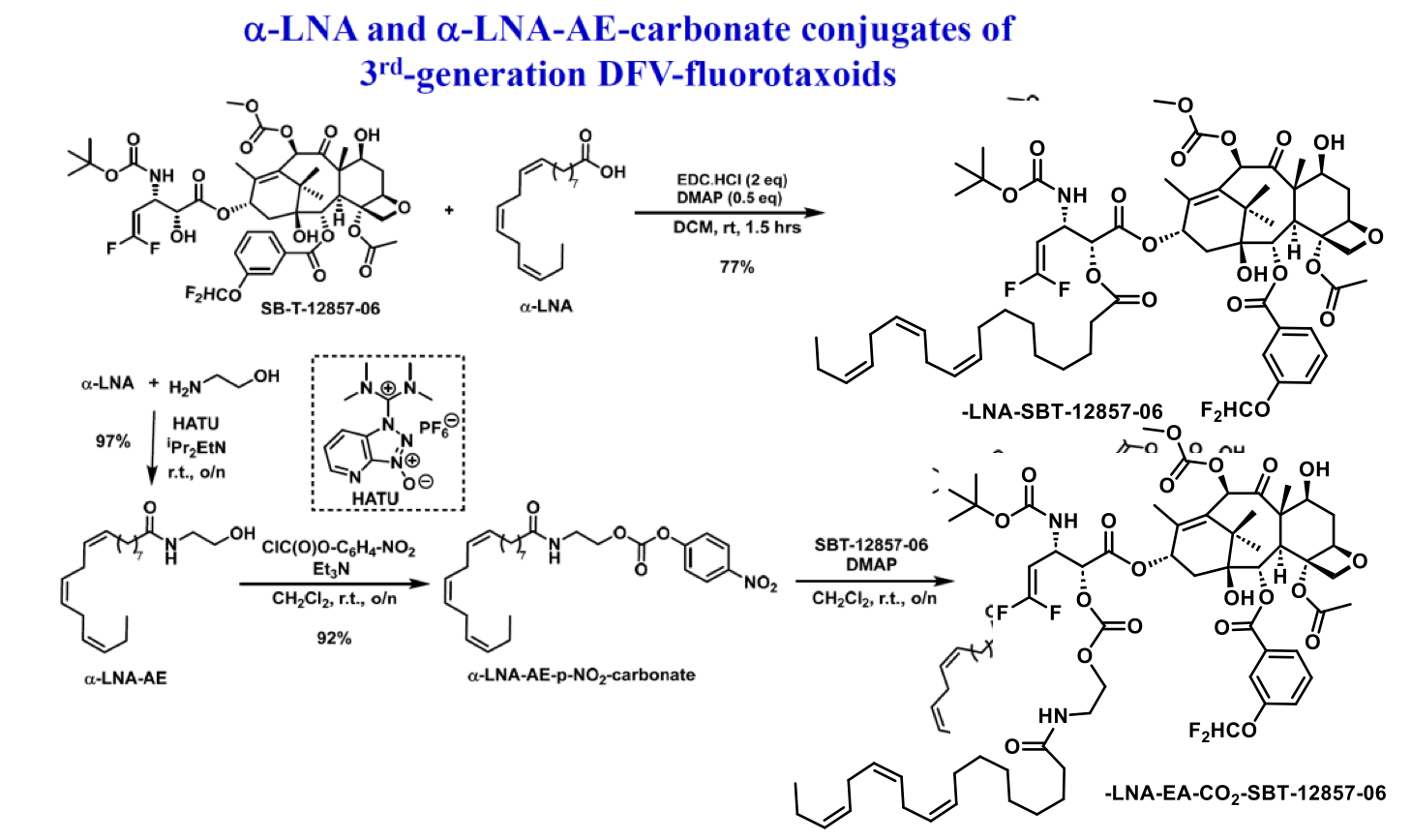
Novel PUFA Conjugates of New-generation Taxoids for LDLR-Targeted Drug Delivery
The Ojima research group has recently developed a novel series of 3rd-generation fluorotaxoids. These compounds have demonstrated significantly enhanced potency against drug-resistant cancer cell lines such as LCC6-MDR and DLD-1, surpassing the efficacy of both paclitaxel and 2nd-generation taxoids. The exceptional potency of these 3rd-generation fluorotaxoids suggests their potential as highly efficacious chemotherapeutic agents, especially when utilized in nano-formulations or as payloads for tumor-targeted drug delivery systems. Accordingly, a series of PUFA-ester conjugates, as well as PUFA-ethanolamine-carbonate conjugates, incorporating α-linolenic acid (LNA) and docosahexaenoic acid (DHA) were synthesized. The conjugates are intended for use in nanoemulsion formulation and nanoparticle-based LDLR-targeted delivery. Two examples are shown below. Evaluations of the efficacy of these drug conjugates are actively underway.