Nature Chemistry: Collisional Alignment and Molecular Rotation Control the Chemi-ionization
of Individual Conformers of Hydroquinone with Metastable Neon
A newly published review article has recently been published in Nature Chemistry coauthored by Dr. Jesus Pérez-Ríos! In this work, Dr. Pérez-Ríos and his collaborators
focus on a central tenet in chemistry: the relationship between the shape of a molecule and its chemical reactivity.
Chemi-ionization (CI) reactions are important in plasmas, planetary atmospheres and interstellar space, reactive collisions with quantum effects, and other energetic environments. In this study, the authors deployed an experiment utilizing electrostatic deflection to separate individual conformers of 1,4-dihydroxybenzene (hydroquinone, HYQ) within a molecular beam. These conformers differ by a single bond rotation (as shown in the figure below), which leads to different dipole moments. These are intersected with another beam containing metastable neon atoms in the excited (2p)5(3s)13P2,0 states (Ne* ) that ionize the molecular collision partner.
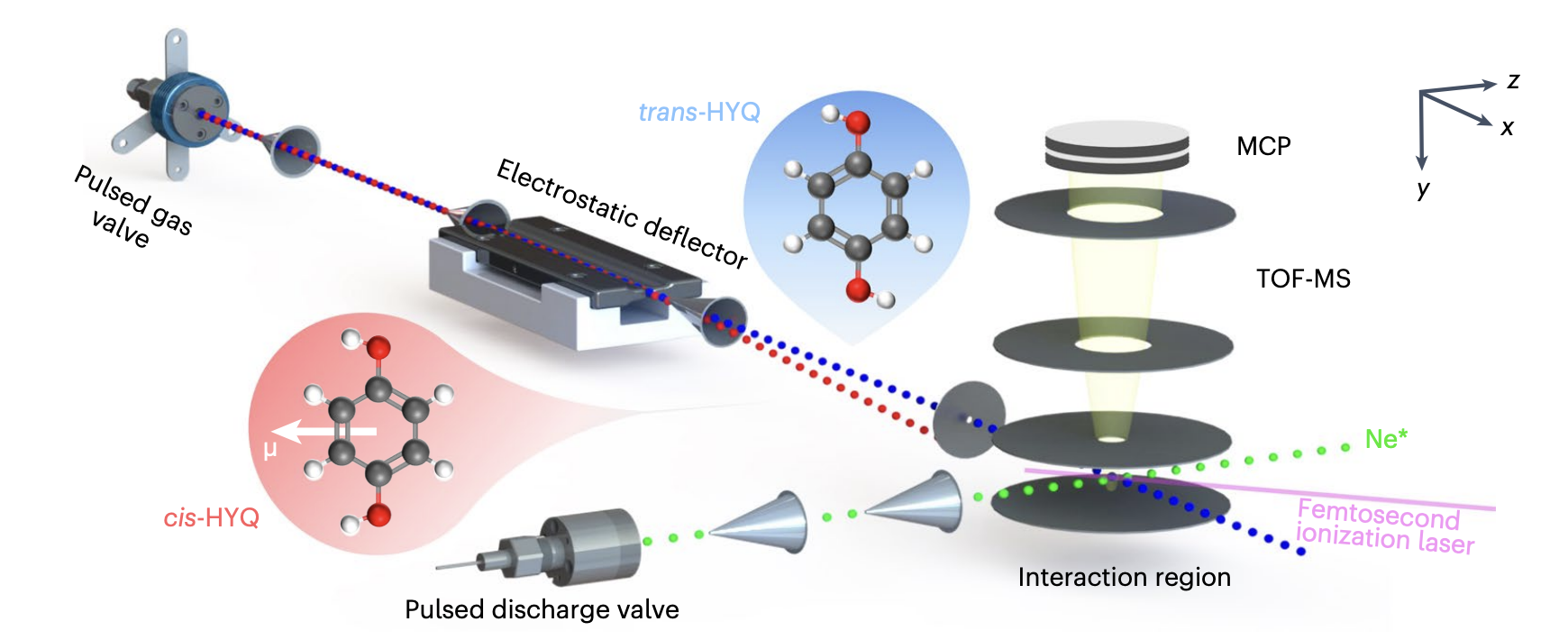
Schematic of the setup and geometries of the polar cis- and apolar trans-HYQ. Differences
in effective dipole moment between the two conformers lead to spatial separation after
passing through the electrostatic deflector.
Spatial separation of the two conformers allows independent analysis of the reactions of each conformer with Ne*. Conformational effects are typically associated with stereodynamics, but that does not necessarily paint the entire picture. Rotational dynamics can also be at play to counter orienting forces. The authors explore the interrelationship between molecular geometry, chemical steering forces and molecular rotation governing CI reactions of specific molecular conformations.
The published article may be found here.