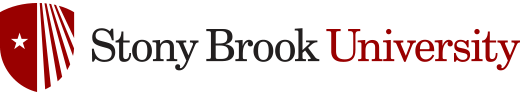Next Generation Taxoids and Taxanes
Paclitaxel and docetaxel have developed into two of the most widely used agents for cancer chemotherapy, with combined sales of roughly 2.8 billion dollars in 2006, however, their lack of tumor-specificity frequently causes the patient to suffer from serious and dangerous side effects. Although they are effective against numerous cancers (e.g. breast, ovary and lung cancers), these usually potent compounds often show little to no efficacy when used to treat melanoma, pancreatic and renal cancers. Resistance to taxane is possible through three different mechanisms: overexpression pf P-gp, mutation in β tubulin binding site and overexpression of the highly dynamic βIII-tubulin isotype.
New-generation taxoids with modifications at position C10 and C13 such as SB-T-1214 can overcome drug resistance caused by those reasons. Furthermore, introduction of a substituent (e.g., MeO, N3, Cl, F, etc.) to the meta position of the C2-benzoyl group of the second-generation taxoids enhanced the activities 2-3 orders of magnitude higher than the parent drugs against drug-resistant human breast cancer cell lines. SB-T-1214 exhibited excellent activity against paclitaxel-resistant ovarian cancer cell lines with mutations in β-tubulin as well, wherein the drug resistance is mediated by the β-tubulin mutation. These taxoids were found to possess exceptional activity in promoting tubulin assembly, forming numerous very short microtubules similar to those formed by discodermolide. Besides, SB-T-1214 exhibited high efficiency against colon cancer in vivo, inducing complete regression of drug-resistant colon tumor xenografts in all surviving mice with tumor growth delay up to 201 days. Such promising antitumor activity of SB-T-1214 led us to suggest that this compound can specifically target tumor-specific CSCs by inhibiting some stemness-related signaling pathways and/or promoting their differentiation.
In collaboration with Dr. Galina Botchkina, we have isolated CSCs from various colon cancer cell lines, induced 3-dimensional CSC spheroids and treated these spheriods with SB-T-1214. Treatment of SB-T-1214 led to a nearly-complete inhibition of the stem cell-related genes and significant down-regulation of the pluripotency gene expression in HCT-116, DLD-1 and HT-29 colon CSCs.
β-Lactam Synthon Method for the Synthesis of Taxoids
In 1992, the Ojima group reported the highly efficient semisynthesis of paclitaxel utilizing the beta-lactam synthon method. Coupling of enantiopure beta-lactams to properly derivatized baccatin scaffolds via the Ojima-Holton coupling protocol has allowed the facile synthesis of paclitaxel analogues. Over the past twenty years, our lab has produced a large number of next-generation taxoids, which exhibit marked improvement in cytotoxicity against both drug-sensitive and drug-resistant cancer cell lines.
Enantiopure β-lactams can be synthesized using two methods: (1) ester enolate-imine cyclocondentation using Whitesell’s chiral auxillary; and (2) enzymatic resolution using PS Amano Lipase
β-lactam synthesis using Whitesell’s chiral auxillary
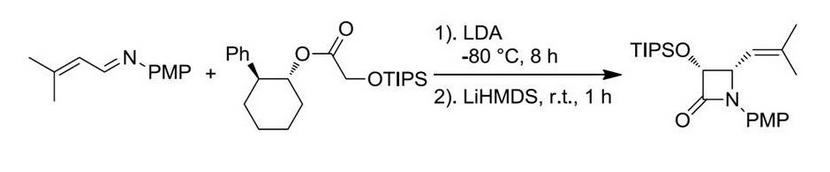
β-lactam synthesis via enzymatic resolution
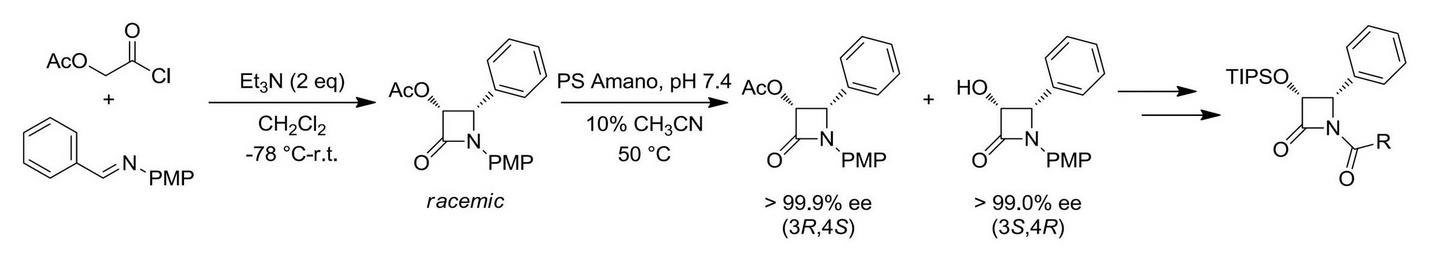
Ojima-Holton coupling
Use of New Generation Taxoids as Warheads
Compared to Paclitaxel and Docetaxel, these new-generation taxoids are 100 times more potent against to P-gp overexpression cells. The new-generation taxoids are also tested in paclitaxel resistant cell lines whose paclitaxel binding site have mutated. Those paclitaxel resistant cell lines are sensitive to all of them. Besides of cytotoxicity, the new generation of taxoids are extremely effective in increasing the rate of microtubule assembly. While βIII-tubulin transfected HeLa cells have a resistance index of 11 against paclitaxel, the resistance index is decreased to 2.5 for SB-T-1102 and to 5.3 for SB-T-1214. Cytotoxicity assay results of 2nd / 3rd -generation taxoids bearing a modified C2-benzoyl group at its meta position are summarized in the Table below. The IC50 values of paclitaxel, docetaxel and19 (SB-T-1214) are also listed for comparison. Majority of these new generation taxoids bearing a t-Boc group at the C3′N position, exhibit remarkable potency against drug-resistant (Pgp+) cancer cell lines, LCC6-MDR, and NCI/ADR, i.e., 2-3 orders of magnitude higher potencies than those of paclitaxel and docetaxel. In more than several cases, the R/S ratios are less than 3 and even become less than 1 in three cases (14g for LCC6 WT:LCC6-MDRand MCF7:NCI/ADR and 15g for LCC6-WT: LCC6-MDR). In these three cases, it can be said that the Pgp mediated MDR is completely circumvented by new taxoids 14g and 15g. In addition to 14g and 15g, 14e and 14l also show excellent R/S ratios, i.e., 14e, R/S ratios of 1.3 and 1.2 for LCC6 WT:LCC6-MDR and MCF7:NCI/ADR, respectively; 14l, R/S ratios of 1.0 for LCC6-WT:LCC6-MDR.

aConcentration of compound that inhibits 50% (IC50, nM) of the growth of human tumor cell line after 72h of drug exposure. bLCC6-WT: human breast carcinoma cell line (Pgp-). cLCC6-MDR: mdr1 transduced cell line (Pgp+). dR/S: resistance factor = (IC50 for drug resistant cell line, R)/(IC50 for drug sensitive cell line, S). eMCF7: human breast carcinoma cell line. fNCI/ADR: multidrug resistant human ovarian carcinoma cell line.
Taxoid Mechanism of Action
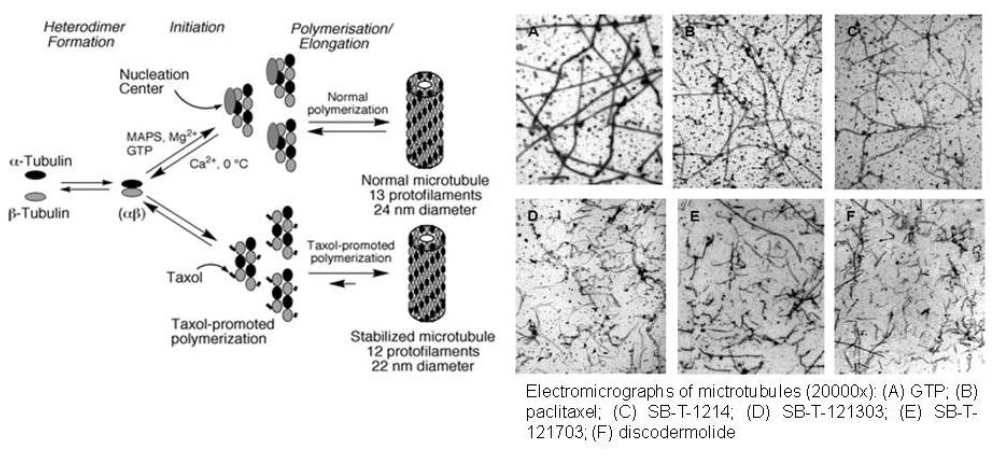
Taxoids are potent antitumor agents which stabilize microtubule polymerization and inhibit the disassembly of the mitotic spindle, stopping chromosome segregation and arresting mitosis in the G2/M phase.These new-generation taxoids interact with microtubules in a manner different from that of paclitaxel and docetaxel.As the figure below shows, GTP and paclitaxel form long and straight microtubules. The microtubules formed with new-generation taxoids are numerous and very short microtubules, in a manner similar to those formed by discodermolide, the most potent naturally occurring microtubule-stabilizing agent.
Genomic profiling of altered clonogenic capacity of HCT116 CSCs after SB-T-1214 treatment
A population of tumor-initiating, or cancer stem cells (CSCs) is responsible for cancer development and exceptional drug resistance, representing a highly important therapeutic target. SB-T-1214 possesses significant activity against prostate CD133high/CD44high tumor-initiating cells. In addition, it induces a previously absent expression of p21 and p53 (“gene wake-up”), which can potentially reverse drug resistance by increasing sensitivity to anti-cancer drugs.
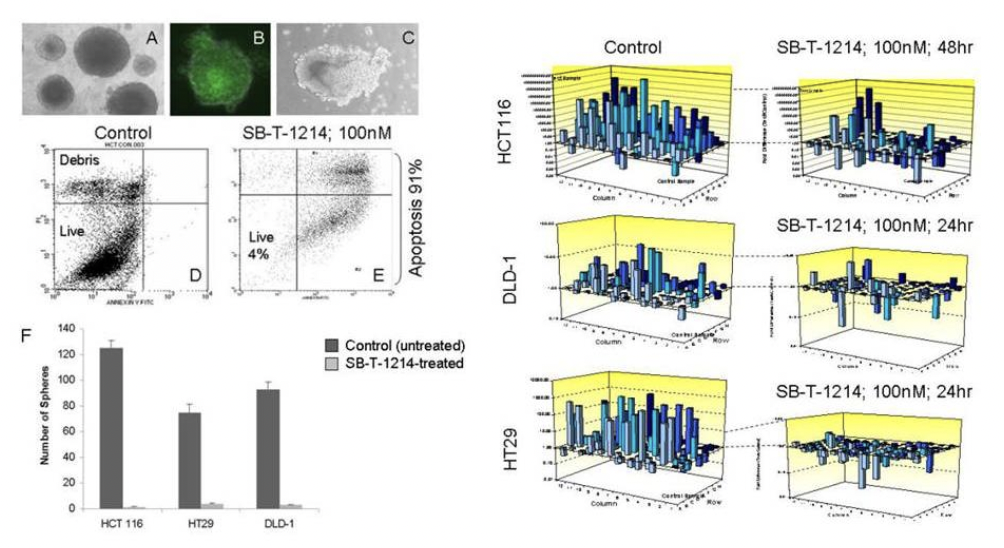
As seen from the figure above, relatively low concentrations of SB-T-1214 (100 nM-1 μM for 24 or 48 hr) induced dramatic down-regulation of stemness in the majority of stem cell-related genes in all three types of colon cancer cells. It is worthy to note that many of these genes were related to the stem cells self-renewal, regulation of symmetric/asymmetric division and pluripotency. For the efficacy displayed in both bulk and CSC-enriched cells, SB-T-1214 was used as drug warheads for our tumor-targeting drug conjugates.
Combination Chemotherapy:Cytotoxic effects of the SBT-1214/CMC2.24 combination against prostate CD133+ cells
Combination chemotherapy involves the sequential delivery of two or more cytotoxic agents based on non-overlapping toxicities and mechanisms of action to augment therapeutic efficacy. Combination chemotherapy decrease in systemic toxicity and increase in efficacy due to synergistic or cooperative effects of the drugs. The combination of CMC2.24 and taxoids are tested against prostate cancer cell line. The result indicated that combination of taxoid and CMC2.24 are more potent than single agent.
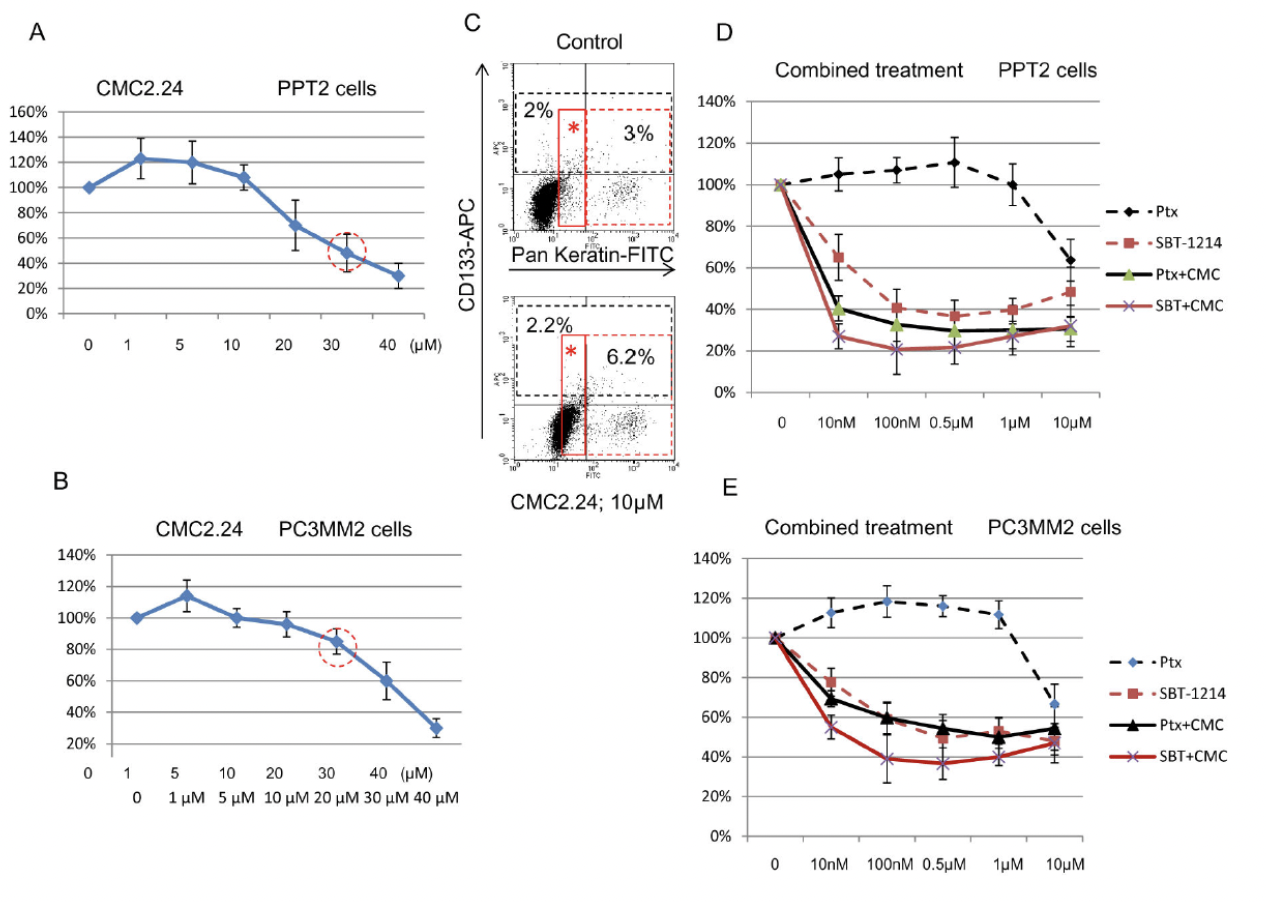
As seen from figure above, lower concentrations of CMC2.24 promoted cancer cell proliferation, while higher ones were cytotoxic. (C) indicated that low concentrations of CMC2.24 increased proliferation of the non-stem progenitors rather than CSCs. A combination of the two agents induced more significant cell death of the CD133+ PPT2 and PC3MM2 cells compared to each compound as a single agent.
Design, Synthesis and Biological Evaluation of 3rd-generation Taxoids
The incorporation of CF3O and CHF2O groups into drug candidates has been shown to enhance their pharmacological properties, including metabolic stability, membrane permeability, and pharmacokinetic profile. New 3rd-generation taxoids bearing 3-OCF3 or 3-OCF2H (with 3-CH3 for comparison) at the C2 benzoate moiety were synthesized and evaluated for potency against drug-sensitive and drug-resistant cancer cell lines. These taxoids demonstrated up to 7 times higher cytotoxicity (IC50) than paclitaxel against drug-sensitive cancer cell lines (MCF7 and LCC6-WT) and 2–3 orders of magnitude higher potency against drug-resistant ovarian, breast, colon, and pancreatic cancer cell lines with MDR-phenotype (NCI/ADR, LCC6-MDR and LDL-1). They exhibited particularly high potency against MDR-cancer cell lines and cancer stem cell (CSC)-enriched cancer cell lines.
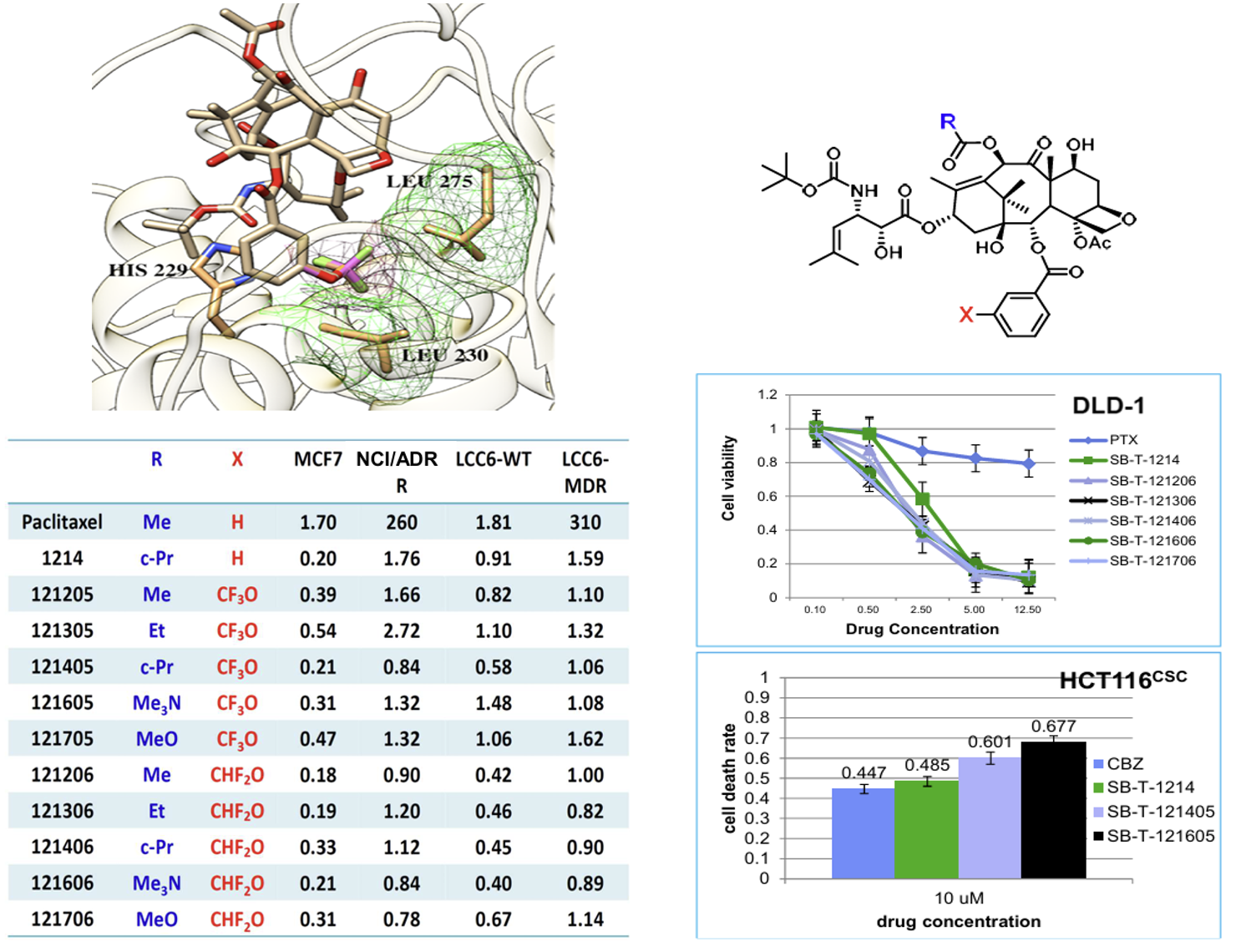
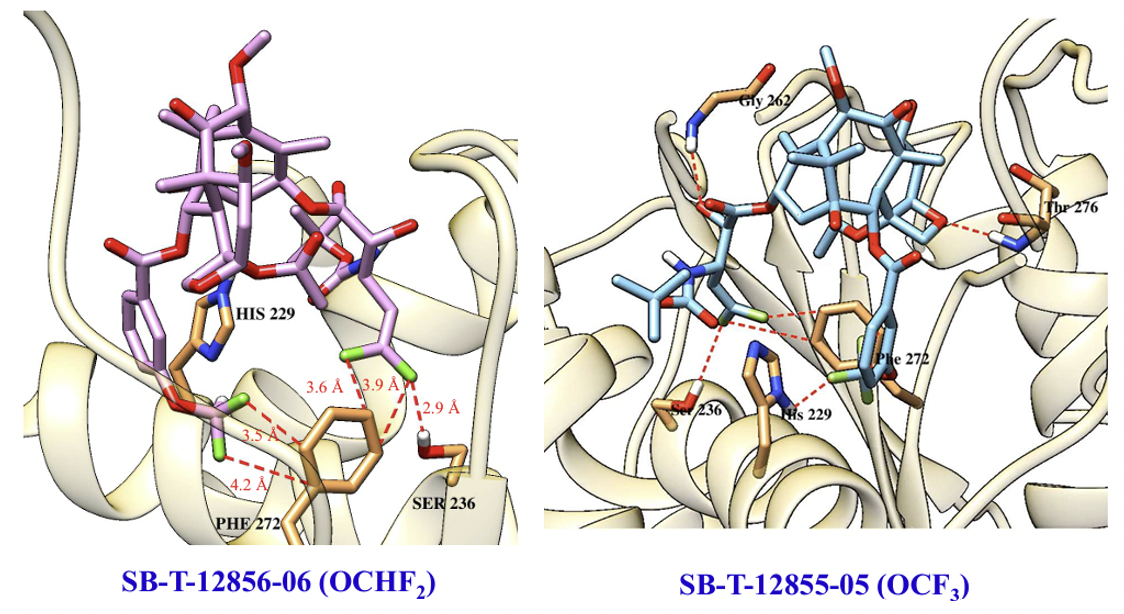
The molecular modeling analysis of the docked structures and predicted affinity of the new 3rd-generation taxoids, SB-T-121205 and SB-T-121206, indicated considerably favorable VDW interactions of OCF3 and OCF2H groups with hydrophobic amino acid residues in the binding site, as compared to Me and OMe groups. The overlay of SB-T-121205 and SB-T-121206 illustrated favorable VDW interactions of the OCF3 and OCF2H residues in the proximal binding site.
Design, Synthesis and Biological Evaluation of Novel 3rd-generation DFV-Taxoids
The incorporation of fluorine or organofluorine groups into drugs often enhances pharmacological properties through unique protein-drug interactions involving fluorine. Previous studies have highlighted the significant effects of the 2,2-difluorovinyl (DFV) group at the C3′ position and the CF3O and CHF2O groups at the 3-position of the C2-benzoyl moiety on the potency and pharmacological properties of 2nd- and 3rd-generation taxoids. Consequently, this investigation combines these two modifications in 3rd-generation taxoids to assess their cooperative effects at the β-tubulin binding site and their impact on the biological activities of the new DFV-taxoids. The new DFV-taxoids showed exceptional cytotoxicity against various cancer cell lines, with subnanomolar IC50 values against A549, HT29, Vcap, PC3, and CFPAC-1. They exhibited 2–4 orders of magnitude greater potency against drug-resistant cell lines LCC6-MDR and DLD-1 compared to paclitaxel, effectively overcoming multidrug resistance.
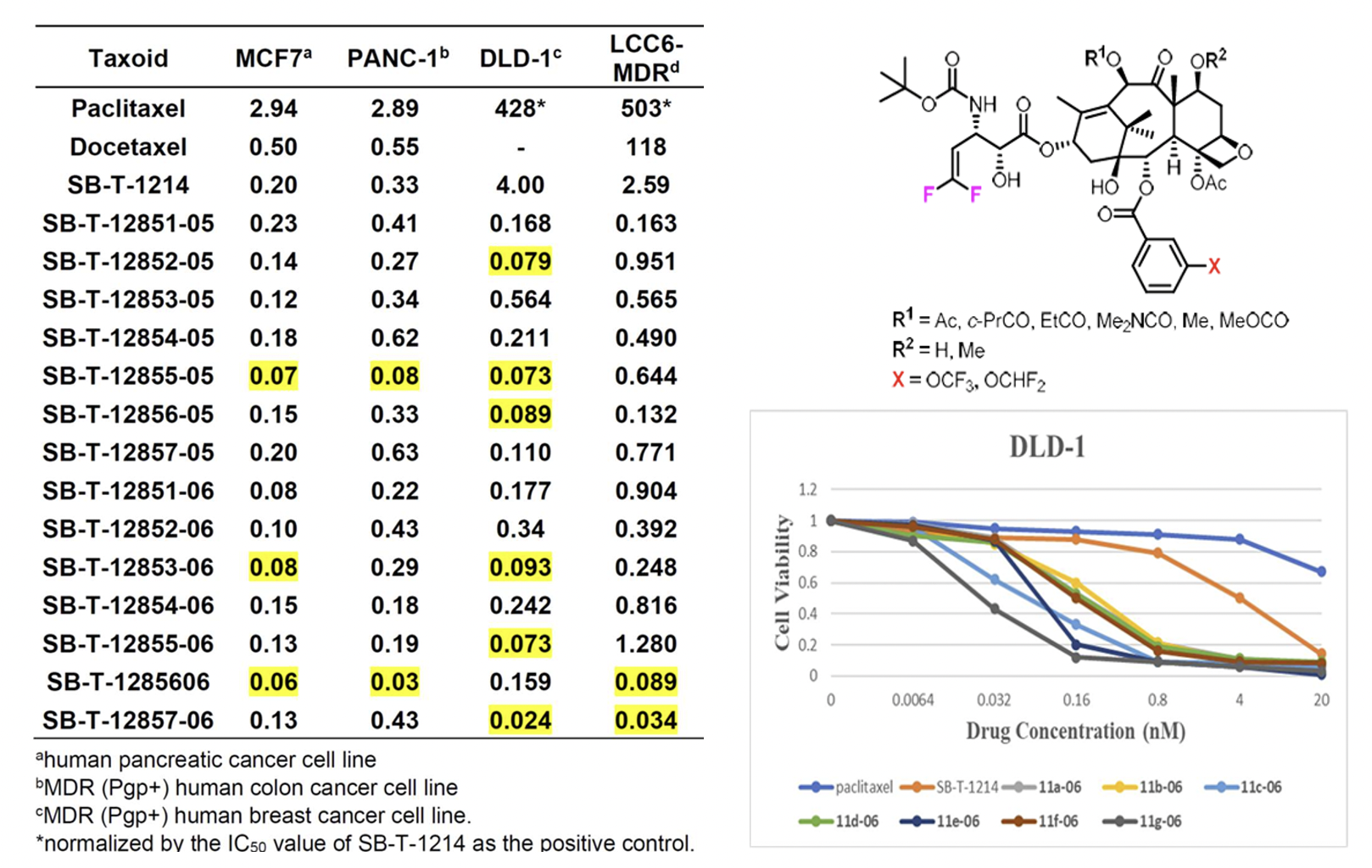
The cooperative effects of the 3′-DFV group and 3-CF3O/CHF2O-benzoyl moiety at the C2 position were investigated using molecular docking analysis. Results showed that both the 3′-DFV moiety and the 3-CF3O/3-CHF2O groups fit well into the deep hydrophobic pocket of the paclitaxel/taxoid binding site in β-tubulin. This enhanced binding mode is facilitated by unique attractive interactions between the fluorine-containing groups and the protein, surpassing the interactions seen with paclitaxel and non-organofluorine-bearing new-generation taxoids. These interactions contribute to the remarkable potency of the new 3rd-generation DFV-taxoids.
Paclitaxel (PTX) is widely used to treat breast and ovarian cancers, but innate and acquired resistance often compromises its applications. This study was to screen new-generation taxanes for their efficiency against both PTX-sensitive and PTX-resistant breast cancer cells. From twelve compounds, difluorovinyl-ortataxel (DFV-OTX) displayed potent cytotoxic activities against both PTX-sensitive and PTX-resistant breast cancer cells. Moreover, DFV-OTX effectively induced tubulin/microtubule polymerization and G2/M phase arrest, leading to apoptosis in both PTX-sensitive and PTX-resistant cancer cells. Molecular docking analysis showed that DFV-OTX possesses unique hydrogen-bonding and van der Waals interactions with β-tubulin. LC-MS/MS analysis also demonstrated that the intracellular drug amount of DFV-OTX was lower than that of PTX, which would be critical to overcome PTX-resistance. Furthermore, DFV-OTX exhibited clear efficacy in the MCF-7R and MDA-MB-231R tumor xenografts in mouse models. Taken together, the results demonstrate that the novel taxane, DFV-OTX, can effectively overcome PTX-resistance in MDA-MB-231R cells, wherein the drug resistance was attributed to ABCB1/ABCG2 upregulation and a distinct mode of action in MCF-7R cells. These results strongly indicate that DFV-OTX is a promising chemotherapeutic agent for the treatment of PTX-resistant cancers.

Related Publications
Wang, C.; Chen, L.; Sun, Y.; Guo, W.; Taouil, A. K.; Ojima, I. Design, Synthesis and SAR Study of Fluorine-Containing 3rd-Generation Taxoids. Bioorganic Chemistry 2022, 119, 105578.
Ojima, I., Chen, J., Sun, L., Borella, C. P., Wang, T., Miller, M. L., Lin,S.,Geng, X., Kuzentsova, L., Qu, K., Gallager, D., Zhao,X., Zanardi, I., Xia, S.,Horwitz,S., Claor, J., Guerriero, J,. Bar-Sagi, D.,Veith, J.M., Pera, P., Bernacki, R. J. Design, synthesis, and biological evaluation of new-generation taxoids. , J. Med. Chem. 51,3203–3221(2008)
Seitz, J., Vineberg, J. G., Zuniga, E. S., Ojima, I. Fluorine-containing taxoid anticancer agents and their tumor-targeted drug delivery. J. Fluorine Chem. 152, 157-165 (2013)
Matesanz, Ruth, Chiara Trigili, Javier Rodríguez-Salarichs, Ilaria Zanardi, Benet Pera, Aurora Nogales, Wei-Shuo Fang Jesús Jímenez-Barbero a, Ángeles Canales , Isabel Barasoain, Iwao Ojima, J. Fernando Díaz "Taxanes with high potency inducing tubulin assembly overcome tumoural cell resistances." Bio. Med. Chem. 22(18), 5078-5090(2014)
J., Sun, L., Borella, C. P., Wang, T., Miller, M. L., Lin,S.,Geng, X., Kuzentsova, L., Qu, K., Gallager, D., Zhao,X., Zanardi, I., Xia, S.,Horwitz,S., Claor, J., Guerriero, J,. Bar-Sagi, D.,Veith, J.M., Pera, P., Bernacki, R. J. Design, synthesis, and biological evaluation of new-generation taxoids. , J. Med. Chem. 51,3203–3221(2008)
Botchkina, G.; Zuniga, E. S.; Das, M.; Wang, Y.; Wang, H.; Zhu, S.; Savitt, A. G.; Rowehl, R. A.; Leyfman, Y.; Jingfang, J.; Shroyer, K.; Ojima, I. New-generation taxoid SB-T-1214 inhibits stem cell-related gene expression in 3D cancer spheroids induced by purified colon tumor-initiating cells. Molecular Cancer,9, 192(2010)
Botchkina, G. I., Zuniga, E. S., Rowehl, R. H., Park, R., Bhalla, R., Bialkowska, Johnson, F., Lorne M. Golub, Zhang,Y., Ojima,I., Shroyer, K., R., Prostate cancer stem cell-targeted efficacy of a new-generation taxoid, SBT-1214 and novel polyenolic zinc-binding curcuminoid, CMC2. 24. PloS one, 8(9), e69884(2013)
Wang, C.; Wang, X.; Sun, Y.; Taouil, A. K.; Yan, S.; Botchkina, G. I.; Ojima, I. Design, Synthesis and SAR Study of 3rd-Generation Taxoids Bearing 3-CH3, 3-CF3O and 3-CHF2O Groups at the C2-Benzoate Position. Bioorganic Chemistry 2020, 95, 103523.
Rong, C. Wang, X. Zhang, Y. Wei, M. Zhang, D. Liu, H. Farhan, S. A. M. Ali, Y. Liu, A. Taouil, W. Guo, Y. Wang, I. Ojima, S. Yang, H. Wang, Cancer Lett.491, 36-49 (2020)